|
 |
 |
 |


Carbon sequestration in the Pacific Northwest:
a model
|
by: Ana Carolina Manriquez
Program Authorized to Offer Degree:
Table of Content** Back to the RTI Theses page **
List of Figures
List of Tables
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
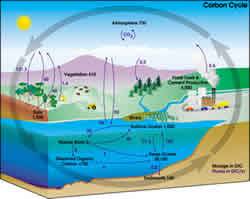
| Figure 1. The carbon cycle on Earth. Illustration from NASA Earth Science Enterprise |
Understanding the factors and processes driving and influencing the cycle of carbon in a particular ecosystem is critical to achieve proper management of the aboveground biomass and soil organic matter, whether it is for reducing greenhouse gas emissions or improving soil quality.
2.1. Forest Ecosystems
Forest ecosystems have essentially three carbon pools: the living
biomass, detritus (debris from dead plants and animals) and soils.
Soils contain almost twice as much carbon as the aboveground vegetation
and the atmosphere carbon combined (Brady 1996). Through the decomposition
and the accumulation of organic matter, soils have a major effect
on the regulation of the carbon cycle. When soil and aboveground
organic matter decline, atmospheric carbon increases, with global
consequences, such as the greenhouse effect. Potential mechanisms
for reducing net carbon emissions through increased carbon sequestration
include the forest ecosystem together with the forest socio-economic
system, with both of those systems dynamic's affecting the carbon
cycle. Conservation and adaptive management of existing forests,
the establishment of new forests (forest ecosystem level) and the
substitution of fossil fuel based energy and products by wood biomass
(forest socio-economic system) could further increase the fixation
of carbon from the atmosphere (Kohlmaier et al 1998).
Forests store carbon as they accumulate biomass, but forests are
also commercial sources of timber and wood fiber. In most carbon
accounting budgets, forest harvesting is usually considered to cause
a net release of carbon from the terrestrial biosphere to the atmosphere
(Houghton et al 1983, Harmon et al 1990). As the debate about controlling
or mitigating atmospheric carbon dioxide concentrations moves from
the study of the scientific issues to a search for practical solutions,
a central question becomes whether commercial use of forests could
be managed to contribute to terrestrial sequestration of carbon.
Can forest management practices be developed so that they meet the
multiple goals of providing wood and paper products, economic returns
from natural resources, and also sequester carbon from the atmosphere?
In managed forests, the amount of additional carbon sequestered
will be determined by three factors: the increase of standing carbon
biomass due to land use changes and increased productivity, the
amount of carbon remaining below ground at end of rotation, and
the amount of carbon sequestered in products and energy, including
their disposal (Johnsen et. al. 2001).
As stated previously, forests represent a huge storage of carbon
since they hold about 80 % of the carbon fixed in the living biota,
and much interest and effort has been put into their study, because
of the possibility of being directly altered by human activity (Apps
& Price 1996). The role of forests, as sink and sources of carbon
in the carbon cycle, is not static at any spatial or temporal scale.
Temporal changes in the forest ecosystem carbon pools are mainly
driven by the dynamics of the carbon pools. Keeping track of the
ecosystem processes, including population dynamics, is a crucial
part of the carbon assessment. This assessment should be done at
the stand level, which is believed to be the appropriate scale for
such analysis (Apps & Price 1996; Harmon 2001). Forest ecosystems
are complex, dynamic and diverse. Forest stands can be complex,
dynamic and diverse. They all have however, three carbon pools:
the living biomass, detritus and soil pools. All of these components
have a role in the carbon cycle dynamics. The soil, a natural body
of organic and inorganic materials and living forms, provide the
substrate for plant growth. Detritus, the debris from dead plants
and animals, is a source of storage as well as a source of food.
The live biomass, which includes above and below ground pools, composed
of coarse and fine roots, understory and canopy, captures carbon
dioxide while releasing oxygen, and also respires, releasing part
of the carbon dioxide previously absorbed.
A more detailed literature review will be given first for soil,
followed by above and below live biomass. Lastly, the detritus component
will be explored, including plant debris (litter fall) and harvest
residuals (slash).
2.1.1 Soils
Conversion of natural to agricultural ecosystems has lead to drastic
perturbations in the processes governing the soil organic carbon
dynamics. Deforestation, biomass burning, plowing, residue removal,
fertilization and single crop cycles have been depleting the earth's
soils in most agroecosystems by 50 to 70% (Lal 1995). The effects
of forest management on carbon soil storage are not as clear nor
as well understood as in agricultural systems. Estimated carbon
storage in below-ground components is known and has been measured
(Brady 1996), but it is mostly how harvesting and management affects
the soil carbon where knowledge is lacking.
Soil carbon has been found to be strongly dependent on the stand
composition and climate (Schlesinger 1977), therefore very hard
to model. Organic carbon in the root zone accounts for approximately
2/3 of the carbon in terrestrial ecosystems worldwide (Post et al.
1982). It is less responsive to harvest than the litter fraction
because of its long residence time. Turn over rates encompass a
large range. Post et al (1982) estimated a turn over rate of 0.00083
per year, although faster turn over rates have also been shown:
0.013 per year (Gardner & Mankin 1981) and 0.025 per year (Schlesinger
1977).
Harvesting can have a significant increase or decrease effect on
forest floor biomass, mostly based on how much slash is left behind
after the operation (Johnson 1992). The majority of studies however,
showed little or no change in the soil mineral carbon after harvest,
with less than 10 % increase or decrease (Fernandez et al., 1989;
Johnson et al., 1991; Aztet et al, 1989, Huntington and Ryan, 1990;
Alba and Perla, 1990; Lawson and Taylor, 1990; Raich 1983). Exceptions
are usually found after harvesting in tropical areas, where soils
are poor and the environmental condition are proper for rapid decomposition.
Houghton et al (1983) developed a global carbon model, in which
the assumption is that after forest harvest, tropical, temperate
and boreal ecosystems loose 35, 50 and 15 % of litter and soil carbon.
Harmon et al (1990) assume no change in soil carbon although noted
that most probably soil organic matter would decrease with intensive
forest management.
Fire, be it a prescribed or a wild fire, will reduce the carbon
and the overall floor biomass, the effects depending primarily on
the intensity of the burning, with the upper 15 cm., the surface
soil, most readily influenced by land use and soil management. In
the Pacific Northwest, a study found significant losses of floor
biomass and nitrogen (40%) after a wildfire (Grier 1975). Another
study, this time on broadcast burning, found a decrease in soil
carbon (20-30%), with an equal or higher increase in the soil carbon
almost two years after the prescription (40-70%) (Macadam 1987).
Carbon soil can be increased with fertilization, because of its
effect on primary productivity. Effects of nitrogen fixation and
fertilization on soil carbon have given results on carbon soil increasing
from 30 to 100 % depending on the site and the species mix composition
(Alnus rubra and Ceanothus spp.) (Binkley 1983; Binkley et. al.
1982). Despite all the unknowns and uncertainties of soil carbon
dynamics and management impact on those dynamics, the commonly held
assumption of soil carbon losses of 30-40% (Musselman and Fox 1991)
after harvesting was not corroborated by the literature review.
2.1.2 Above ground living biomass
Different studies present a dichotomy on aboveground biomass dynamics,
with some suggesting aboveground components can be a net sink (Delcourt
& Harris 1980, Oliver et. al. 1990) or a net source (Houghton
et. al. 1983; Harmon et. al. 1990) of carbon. Both cases are correct.
The analysis and assertions on what an ecosystem's carbon is or
will become under a certain line of management will depend on what
was the state of the ecosystem before any management was conceived.
Furthermore, it will depend on how extensive is the spectrum under
which carbon cycling is considered. For example, Harmon et al (1990)
argued that the conversion of old-growth forests to younger forests
under current harvesting and use conditions has added and will continue
to add carbon to the atmosphere, even when considering long term
products such as lumber. Oliver et al (1990) found similar results
at the forest ecosystem level, but further argued the conversion
of old growth to managed stands is negligible when compared to the
addition of carbon by the burning of fossil fuels. Similar results
were found by Schlamadinger and Marland (1996). This is why it is
very important to establish first and foremost the spectrum under
which the carbon accounting story will be evaluated. Nobody would
deny that an old growth stand stores more carbon at the forest level
than a younger stand, and that the younger stand has a greater primary
productivity, with higher rates of yearly uptakes of carbon. With
these differences taken into consideration, the above ground living
biomass is further analyzed.
In the development of a forest, the foliage, litter fall, net primary
production and nutrient accumulation in above ground tree components
usually reach a plateau at the stem exclusion stage (Tadaki 1966,
Gessel & Turner 1976, Oliver 1981, Sprugel 1985). This trend
seems to be true for Douglas-fir as well (Turner & Long 1975)
and directly impacts the development of biomass through time in
the different components.
The distribution of standing forest biomass in representative stands
in the Pacific Northwest region has been previously estimated (Grier
& Logan 1977, Keyes 1979, Edmonds 1980, Vogt et al 1980, Gholtz
1982, Cooper 1983, Keyes & Grier 1981, Santantonio & Herman
1985, Vogt et al 1986, Edmonds 1987). The total biomass and forest
carbon will depend on the stand conditions, its age, density, species
composition, etc. However, the patterns of biomass distribution
in conifer stands of the forests of the Pacific Northwest are very
similar and roughly as follows: 65-75% in the stem and bark, 15-20
% in coarse roots, 5-10 % in the crown (branches and foliage). Biomass
in stem and bark on a 40 year old Douglas fir stand on a high productivity
site was about 76 % (Cooper 1983), and this proportion was about
73 % in a low productivity site planted with Douglas-fir (Keyes
& Grier 1981). Similar values have been established for old
growth Douglas-fir in western Oregon (Grier and Logan 1977).
Looking at the components on conifer stands separately, a nearly
complete foliage cover is established early in stand development
of most forests and remains essentially constant until maturity
(Grier & Logan 1977, Keyes 1979, Cooper 1983). Branches, as
extensions of the stem, can accumulate carbon through the life of
the tree. The fraction of biomass in branches is usually higher
for hardwood stands, with as much as 25 % of the biomass found in
that component. This proportion is much smaller for conifer trees,
with about 5-7 %. The stem biomass increases rapidly with age while
the foliage biomass stays fairly constant (Grier and Logan 1977).
Carbon content is approximately 50 % of the oven dry weight (Reichle
et al 1973, Harmon et al 1990) with slight differences related to
the chemical and physical composition of some of the components
(Vogt 1991).
2.1.3 Below ground living biomass
The importance of roots as structural, storage and physiological
organs has been acknowledged for quite some time (Harris 1971, Santantonio
1977). However, they have not been, for the most part, included
in ecosystem research because of the difficulties surrounding their
study. Observations are not possible without major disturbances
in the soil, while changing dramatically the environment of the
roots.
The development and buildup of the roots biomass is more complex
than some of the above ground components. This is due to the variety
of roles played by coarse and fine roots: structural support, food
storage and nutrient absorption for example. However, in their 1992
study on spatial disposition and extension of the structural coarse
root system of Douglas-fir, Kuiper & Coutts found significant
positive correlations between all the coarse root parameters studied
and the tree diameter at breast height (dbh). Furthermore, data
on the relationship between coarse root biomass and dbh in Douglas-fir
in the Netherlands was found to be consistent with natural stands
of Douglas-fir in the Pacific Northwest (Santantonio et al. 1977),
even though the site conditions and management history between the
two sites were very different. Dbh, which is readily available,
has therefore been shown to provide good estimates for woody root
biomass.
Decomposition rates for woody roots in forest ecosystems of the
Pacific Northwest were estimated by Chen et al. (2001), with Douglas-fir
roots having an estimated decomposition rate of 0.05/year for roots
between 4 and 12 cm.
Fine roots on the other hand are very hard to account and simulate
based on growth models. An extensive study on fine root biomass
related to stand age and productivity found no significant differences
among stands of different age but same site productivity (Vogt et
al 1987). Another study did a sensitivity analysis dealing with
the incorporation of fine roots biomass into the soil carbon, leading
to the assumption of fine roots flux being relatively constant (Cropper
and Ewel 1984). In biomass studies and budget estimations, fine
roots biomass estimates from previous studies are added to the total
estimated by the simulations (Harmon et al 1990, Keyes & Grier
1981), or total root biomass is based on a percentage of the bole
(Bruschel 1993), but none of the studies from the literature reviewed
provided a potential way of simulating their growth and death.
2.1.4 Forest floor
Carbon accumulation in detritus and soil often accounted for greater quantities of biomass than the living biomass, especially on hardwood stands (Schlesinger 1977, Covington 1981, Gholz & Fisher 1982, Moore & Braswell 1994). The return of organic litter to the forest floor is complex and very variable. Factors to consider among others are: the age of the stand, the species composition, the density of stand, the site productivity and the environmental conditions (Bray & Gorham 1964). It is clear that litter-fall plays a fundamental role in soil formation and site productivity (Bray & Gorham 1964, Schlesinger 1977, Covington 1981, Gholz & Fisher 1982, Moore & Braswell 1994). It is also clear that both the carbon chemistry and nutrient concentrations of litter strongly affect its decomposition (Aber et. al. 1990). Thus, detrital mass changes more rapidly than soil carbon with disturbances.
The amount of change when harvest occurs will be highly dependent upon the harvesting method, the stand composition and the climatic conditions (Cooper 1983). Harvesting usually increases decomposition rates of the detritus material because it causes higher soil temperatures and moisture, together with increased availability of inorganic nutrients needed by decomposers (Aber et al 1978). Temperature and moisture variables have been found to be the main factors explaining decomposition patterns, stronger when considering them together rather than individually (Gholtz et al 2000). Turner and Long (1975) showed that leaf litter (which has the highest concentrations of nutrients and decomposes faster) decreases in time, but total tree litter increases in time because of returns of less decomposable woody litter. Similar results were found in old growth Douglas-fir ecosystems, where woody material represented about 60% of the biomass returns (Grier et al 1974). A study on Douglas-fir stands ranging from ages 22 to 160 showed that a typical leaf litter production is 2 MT/ ha/ year, while total litter is in the ranges of 2.5 MT/ ha/ yr (Gessel & Turner 1976). Annual fall of litter increases until about age 40, and then becomes relatively constant while total litter continues to increase because of woody litter, although it can be very irregular.Dimock (1958) showed what intuitively seems correct with regards to thinning operations: decreasing levels of litter fall with increasing intensity of thinning regimes on Douglas-fir stands.
The decomposition of coarse woody debris although little understood, is a very important aspect of nutrient cycling in forest ecosystems of the Pacific Northwest (Harmon 1992). Turner and Long (1975) calculated decomposition rates for an age sequence of Douglas-fir stands. The decomposition rate starts at about 0.05 /year for a young stand, and increases to about 0.16 /year at age 30, decreasing to about .1 /year at age 50 and above.
2.1.4.2. Logging debris: slash
Slash burns are very rarely done anymore and have not been done
for most of the last 20 years because of smoke. On the west side
of Washington Cascades, on slide ground, the slash is left unburned
unless whole tree yarding is the harvest method. In the case of
whole tree yarding, logs are processed by a delimber and slash is
burned on the landing. On gentler terrain, where the cut-to-length
system is used for thinning, slash remains unburned. When shovel
logging is the harvest technique for a clearcut, burn piles are
created and combustion is fairly complete (Mason, personal interview,
11.2001).
Harvesting can have a significant increase or decrease effect on
forest floor biomass, mostly based on how much slash is left behind
after the operation (Johnson 1992).
2.2 Managing for carbon sequestration: the Silviculture
2.2.1 Longer rotations
Long rotations develop structurally complex managed forests and increase the accumulated timber volume per unit area (Franklin et al 1997, Burschel et al 1993). Longer rotations are ecologically viable because Douglas-fir (Pseudotsuga heterophylla) and other associated conifers can live to a very old age and their productivity is maintained to advanced ages (Curtis 1997). Longer rotations should be combined with thinning regimes to increase the productivity and the size of trees in a shorter time span. Larger trees imply higher wood quality, and the thinning regimes can provide revenue as intermediate operations. Longer rotations allow for adjusting unbalanced age distributions, increasing the quality of wildlife habitat associated with late successional forests, and increasing the net standing carbon storage capacity.
2.2.2 Variable retention
The variable retention system (Franklin et al 1997) is based on
the concept of retaining structural components of a particular stand
for at least another rotation. The development and maintenance of
a structurally complex forest is the most important point when talking
about the restoration of a forest. It is very flexible and the level
of retention directly relates to the management objectives. It is
important to consider other functions of these structural components,
beyond the carbon sequestration per se, such as the enriching attributes
and enhancement of connectivity throughout the landscape. The idea
is to provide structural elements for diverse habitat requirements,
ameliorate the microclimatic conditions, and maintain microfauna
(mycorrhizal fungi, lichens etc). Enriching stand structure by maintaining
living and dead structural material of various sizes, species, and
levels of decay through aggregated or dispersed retention can also
be incorporated into the management of the forest. Leaving behind
coarse-woody debris following thinning and harvesting operations
is recommended to increase the carbon in the forest floor.
The management objectives will determine what will be retained,
how much and in what pattern. Large trees with special features
such as rot pockets, cavities and large limbs or clusters of limbs
should be retained. Snags in different states of decay and sizes,
as well as coarse woody debris in different sizes and stages of
decay should also be retained. The pattern in which these structures
are to be left will depend on the stand and its characteristics.
Aggregated retention will be preferred at some points, and dispersed
retention will be the choice on others, hopefully through the mixture
achieving greater complexity and carbon sequestration. Shelterwood
(Smith et al 1996) for example, is a type of dispersed retention
of dominant and co dominant wind firm and stress tolerant trees,
that will provide in time a well distributed source of snags and
coarse woody debris.
2.2.3. Thinning regimes
Thinning can be used to promote the overall health of a forest,
through reduction of high fuel loads and increased wind stability.
Thinning can be used to salvage material from disturbances and avoid
insect outbreaks (Smith et al 1996, Oliver & Larson 1996). This
is an important consideration when addressing issues such as fire
safety, insects, wind stability and diseases. More important however,
thinning can be used to accelerate the stand dynamics of a particular
stand, favoring certain structural components that have a functional
value, releasing growing space for understory species and advanced
regeneration, or simply to increase the size of trees. Thinning
in restoration is used as a tool that affects the structure of the
stand. Pre-commercial thinning (PCT) is applied near the end of
the stand initiation to enhance survival, growth and value of the
residual trees. It increases stand uniformity but promotes tree
growth and understory development (shrub and herbaceous) allowing
also for early establishment of shade tolerant species (Oliver and
Larson 1996). By doing a PCT, the differentiation of the stand is
accelerated and the structural and species components increased.
The spacing can vary in patches through the plantation, with small
openings or gaps created to retain components of the early initial
stage.
Thinning combined with extended rotations can maintain forest cover
for long periods while still providing wood products, through allowable
intermediate operations; timber flow can be sustained during intermediate
stages of development with the benefit of ecological processes being
maintained and higher wood quality achieved (Oliver 1993, Burschel
et al 1993).
2.3 Accounting for the sequestered carbon
The Kyoto Protocol to the United Nations Framework Convention
on Climate Change (1998) prescribes that net flows into or out of
the biosphere will be represented by the changes in carbon stocks.
This notion simplifies the measurements and accounting processes.
The Intergovernmental Panel on Climate Change (2000) is consistent
with this prescription, defining carbon sequestration as an increase
in carbon stocks anywhere but in the atmosphere. The important issue
is "additionality" (Chomitz 2000). Additionality addresses
the idea that carbon sequestration or reduced emissions can result
from a management change. Management alternatives can be compared
against a base line, to measure the change from "business as
usual". Afforestation of grazing land for example, is a one
time huge addition of carbon pools and if reforested after disturbance,
the carbon pools can be maintained through a long period of time.
How do we measure carbon and how can we estimate the variations
in the different terrestrial pools? Biomass is one of the key characteristics
of forest ecosystems because it contributes in the definition of
carbon flux and nutrients, as well as the potential standing and
dead organic matter in a particular site. Biomass studies are essential
for understanding ecosystem dynamics. Biomass studies are static
however, describing and estimating living and dead material in a
particular stand at a particular time (Santantonio et al 1977).
Combining biomass studies with growth models seems to be the most
straightforward manner for estimating component masses at different
points in time at the stand scale. The carbon storage pattern simulated
by the model is static, meaning productivity of site is assumed
constant as embedded in the original inventory in question, without
possible changes associated to different temporal scales, like the
global warming issue. The Kyoto Protocol specifies integration of
greenhouse emissions with corresponding offsets credits if carbon
is removed from the atmosphere on a 5-year commitment period. Integration
over spatial scale might be used as well to decrease the costs in
accounting, monitoring and verification.
2.4 Forest products, biofuel and substitution
Harvesting of forest ecosystems changes the natural carbon cycle
between the terrestrial pools and the atmosphere. Therefore, the
balance between forests and forest products is an important component
in any budget analysis and should be included.
The carbon fluxes related to the harvesting activities should follow
the general equation for atmospheric flow (Winjum et al 1998): net
carbon flux to the atmosphere = carbon fluxes to the atmosphere
from harvesting activities and forest products - carbon sequestration
during development of the forest. The carbon fluxes associated with
forest harvesting activities and the use of wood should include
the carbon emissions from decomposition of slash left in the forest
after harvest, the burning of fuelwood, the waste from manufacturing
wood products, and the decay of the products pool.
Over a long term period, the amount of carbon stored in the biosphere
reaches a steady state, and continuing mitigation of carbon emissions
depends on the degree fossil fuel use is displaced by biofuel and
wood products (Schlamadinger & Marland 1996).
A modeling study comparing the growing of trees to sequester carbon vs. growing trees to substitute fossil fuels showed that many factors influence this trade off. Important variables were: forest productivity, the efficiency of the production of wood based energy, previous land uses and the time scale considered (Marland & Marland 1992). The benefits in terms of using wood as energy instead of leaving it in terrestrial pools of storage increased with increased productivity of the site, with greater time intervals considered and with the efficiency and usage given to the biomass products.
There is also the idea that forest products require less energy in their manufacture than other products for the same use. If wood products can replace more energy intensive products with the same function, the substitution by wood products will also provide a decrease in the carbon emissions to the atmosphere (Koch 1991). Aluminum, steel, cement, bricks and synthetic materials derived from fossil fuels cause a greater energy consumption and greater carbon additions to the atmosphere. For example, the net energy required per ton of lumber studs is 2.91 million BTU (oil equivalent). The net energy required per ton of steel studs is 26.67 million BTU (Koch 2001).
The Intergovernmental Panel on Climate Change (1990) recommendations and guidance with regards to response strategies in the forest management context included the replacement of fossil energy sources by sustainably managed sources of biomass, increase substitution efforts of highly energy consuming products by wood, technology improvement with regards to the use of fuel wood, and encouragement of the recycling of forest products to provide even longer storage for carbon pools.
2.5 Carbon credits
The Kyoto Protocol to the United Nations Framework Convention on
Climate Change (1998) has proposed a way for establishing limits
on greenhouse gas emissions to be enforced internationally, with
different types of commitments for developed and developing countries.
The protocol allows, within a set of rules, for countries to use
their terrestrial sinks to offset part of their greenhouse gas emissions
from other sources.
The idea of emission trading has also been included in the Kyoto
protocol. Countries listed in Annex B of the protocol can offset
their own emission reduction commitments by engaging in emission
reduction activities in another Annex B country (developed) or a
non Annex B country (developing). The protocol is however unclear
if carbon sequestration can be used the same way the emission reductions
activities are carried between Annex and non Annex B countries.
Among other issues to be resolved before the Kyoto protocol can
be implemented internationally and commitments enforced internationally,
is that accounting rules for emissions and reductions need to be
tested and put in place (Marland et al 2001).
Birdsey and Heath (1997) estimated on a large scale assessment that over the past 40 years, US forests have sequestered enough carbon to offset approximately 25 % of the current US emissions. Creating a market for reducing carbon dioxide emissions through forest sequestration requires three elements: a market framework, demand from willing buyers and supply from willing sellers. For a market framework to be successful, it requires a policy and political framework. Willing buyers come from consumers interested in reducing their emissions. They need insight about the options that forest conservation and management provide in terms of reductions and mitigation. The third part has to be about providing the supply: landowners understanding the carbon dynamics of their forest, how to increase it through management and how to access the markets.
One last point: trying to protect the global climate through carbon sequestration, by coming up with efficient accounting mechanisms that encourage carbon sequestration in forests and forest soils also provide incentives for other desired activities such as the sustainable management of natural resources and protection of biodiversity.
3. METHODS
A prototype carbon sequestration analysis model was developed for the West Cascades of the Pacific Northwest region. It is to be used with tree list inventory data and growth and yield model simulations of inventory conditions. Microsoft Excel was selected for use as the spreadsheet program with which to build the carbon storage model. The model was designed to be easily adapted and updated. Certain carbon factors can be very specific to a particular area and new knowledge is constantly being acquired on the carbon cycle and its components and should be considered if pertinent. Due to the complexity of the carbon cycle and to the accuracy of the carbon model created, carbon storage evaluations are done at the stand level (Apps & Price 1996; Harmon 2001). The complexity of carbon accounting, and the success estimating a carbon balance for the system, increases with the increase of the spatial and temporal scales: the more complex the hierarchy of the system, from individual tree to the landscape level, the more complex its representation and estimation.The carbon model is an attempt to develop a closed carbon model in which all flows into and out of the system are mathematically accounted for. It is at the input and output level where simplifications were made so that forest carbon balance questions could be defined. It is assumed the forest occupies an area of uniform site quality. Also, that changing climatic conditions and CO2 concentrations do not affect processes and their rates, and that repeated harvesting does not reduce long term site productivity.
There are two main parts in the carbon model: the forest module and the product module. The displacement and substitution analysis are derived from the products module.
A critical aspect of the model is that it has to be functional and applicable to a wide array of forest management scenarios and stand conditions. For the forest module part of the model, which is the base of the carbon model, the data required is taken from the Landscape Management System (LMS ) (McCarter et al 1998). LMS is an evolving software application developed at the University of Washington- College Of Forest Resources- Silviculture Laboratory. LMS is designed to assist in landscape level analysis and planning of forest management alternatives. It is implemented as a Microsoft Windows (TM) application that coordinates the activities of other programs (projection models, visualization tools, etc.) that makeup the overall system. Since LMS is modularly designed, it can accept many growth model alternatives for use with simulations.
LMS was utilized to perform projections of inventory conditions providing a broad spatial and temporal context for carbon storage evaluation at the tree and stand levels over a variety of growth and treatment periods. For the PNW forest simulations, the PNW variant of the Forest Vegetation Simulator (FVS ) (Wykoff et al 1982) was selected as the growth model for use within LMS. FVS is a distance independent growth and yield model based on individual tree records. These tree records (diameter, height crown ratio, TPA, etc.) will define growth for small and large trees, as well as mortality, which is density dependent. FVS allows modification of the response of the growth model, making it possible to adjust for specific stand conditions. The PNW variant applies to 37 species from the region. It can portray single or mixed species, even and uneven aged, on a wide variety of forest types. The simulations and analysis are concentrated on conifers and specifically within the Pseudotsuga menziesii and Tsuga heterophylla (Franklin and Dyrness, 1973) forest types.
3.1 The Forest carbon
The carbon forest module includes the following components: branches
(dead and live), foliage, stem and bark, standing dead trees (snags),
coarse roots and litter (harvest slash, dead branches and foliage).
Forests are considered a standing pool of carbon at any point in
time.
The forest module of the carbon model is based on accounting for
all allocations through biomass estimates at discrete points in
time, which establishes where and how much is sequestered in what
components. This allocation changes in time through losses by decomposition,
and harvest operations that use fossil fuels.
Carbon additions or reductions to atmospheric pools resulted from
forest growth, silvicultural treatments and decomposition. These
additions (sequestration) and reductions (emissions) were calculated
as the difference between total estimated forest carbon storage
the growth period before treatment and total estimated forest carbon
storage for the growth period post treatment (Figure 2).
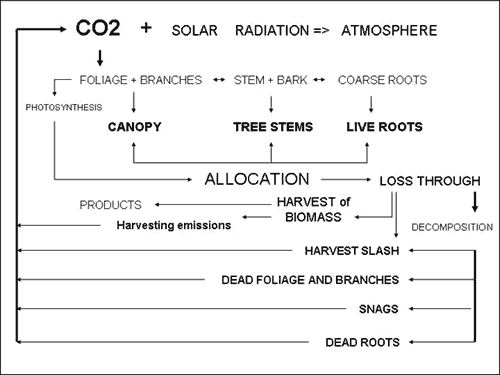 |
| Figure 2. Forest module based on carbon sequestration (additions) and carbon emissions (reductions). |
Soil carbon changes were ignored due to the complexity
of assessing carbon budgets through time after silvicultural operations,
and from the leaching of organic and inorganic carbon at that level
(Harmon et. al. 1990). Research attention has been given to below
ground processes, respiration and foliage dynamics (Landsberg et.
al. 1991), and as information becomes pertinent should be integrated
to the system.
The model has been developed for 5 year growth periods, but LMS
allows for an increase of this time for operations assessed with
10-year growth periods, in which case equations would account for
this change.
The first step is to convert the LMS scenario tables for the forest,
the cut and the snag inventory from English to metric units to be
consistent with the units required for the regression equations
used. The scenario table shows individual tree records with its
respective attributes for all the management periods considered.
Regression equations developed by Ghotlz et al (1979) were used
to estimate tree component dry weight biomass based on diameter
at breast height (d.b.h.) for branches, foliage, stem, bark, snags
and coarse roots in kg/ ha. High correlations are usually found
in logarithmic regressions of dry weight on d.b.h. According to
Bunce (1968), this is in part due to the balance between apical
and radial growth, and because logarithmic units represent progressive
orders of magnitude. The estimation of current and total foliage
biomass using d.b.h. has been shown to have errors in the regression,
especially in older stands, and this should be taken into account
(Grier & Waring 1974, Snell & Brown 1978, Marshall &
Waring 1986). All results however, are benchmarked against biomass
estimates found in the literature (Grier & Logan 1977, Keyes
1979, Edmonds 1980, Vogt et. al. 1980, Gholtz 1982, Cooper 1983,
Keyes & Grier 1981, Santantonio & Herman 1985, Vogt et.
al. 1986, Edmonds 1987, Vogt 1991). The equations, unless cited
otherwise, follow the form:
(1) ln Y = a + b ln X,
where a and b are regression coefficients, Y is the dependent variable and X is the independent one. The equations are species and component specific and have been used in several biomass studies in the region to determine dry matter production (Grier and Logan 1977, Gholz 1982, Cropper and Ewel 1984, Vogt et al 1987, Harmon et al 1990, Canary et al 1996). Derived from equation (1), the equations for biomass (B) follow one of the three forms, depending upon species and component (Appendix A):
(2) B = e b0 * dbh b1
(3) B = b0 + b1 * dbh 2 * ht/100 - b2 * (dbh 2 * ht/100) 2
(4) B = b0 + b1 * (dbh 2 * ht/100)
where b0, b1 and b2 are regression coefficients that are species and component specific (Ghotlz et al. 1979). Standing carbon was estimated by multiplying the biomass output by a proportion factor that depends on the species and component, but averages 50% of dry weight (Reichle et al 1973, Harmon et al 1990, Birdsay 1992). The carbon output is then summarized into three groups: stem (bark and trunk), crown (foliage and branches) and soil (coarse roots). The understory carbon pool represents approximately 1% of forest carbon (Turner at al 1995). Since this is a small percentage of the total forest carbon pool and because models are not available to link understory biomass to tree inventories, estimations of understory carbon storage were not included in this project.
The woody debris pool consists of snags, dead coarse roots and litter fall. The largest pool of organic carbon in most forest stands is soil organic matter and detritus (Schlesinger 1977). Litter mass changes more rapidly than soil organic matter. For the purpose of this project no loss of soil carbon was assumed due to harvest as indicated by three major studies (R. Boone et al 1988, Harmon et al. 1990, Johnson 1992). It is also assumed that the carbon flux of fine roots is balanced: fine roots grow and die at the same rate (Santantonio et al. 1977, Cropper & Ewel 1984). Therefore, organic soil carbon was determined to be relatively constant and was not included in the overall equation for carbon pools.Litter fall was defined as a variable percentage of the total foliage and branches biomass, with an average of 10% of the foliage and dead branches total biomass assumed to accumulate in the litter pool for the 5-year growth period (Franklin and Spies 1988, Edmonds 1979, Grier and Logan 1977). The litter fall pool increases after each treatment because foliage and branches are assumed to be left scattered on site. This means that on the west side on steeper slopes the slash is left unburned unless whole tree yarding is the harvest method. On the gentler terrain where the cut-to-length system is used for thinning, slash remains unburned. Again, for simulation purposes, foliage and branches are considered to decompose on site instead of being burned.
Root biomass of harvested trees was also accounted for and decomposed through time, adding this biomass to the live root biomass pool.
Snags were determined by the tree mortality predicted by the FVS growth model. The general equation for calculating snag biomass (SB) uses species specific equations for live trees corrected for density (Canary et al 1996):
(5) SB = (biomass of live tree stem (Gholtz et. al. 1979) * density of snag (Spies
1988)) / density of live tree (Hartman et al. 1976)
The snag carbon content was estimated by multiplying the snag biomass
times the species carbon factor, which is very close to the live
tree carbon factor. The change in carbon content with regards to
the biomass of the component remains relatively constant between
live and dead trees (Sollins et al 1987). Existing stumps were not
considered in the carbon pool, because data on those components
was not available for calculation.
The reduction of the different biomass pools, such as snags, litter
fall and coarse roots were estimated by decomposing them according
to species specific annual decomposition rates developed by Harmon
(1993)(Appendix A) based on the literature (Harmon et. al. 1986).
They have been evaluated and used by major studies (Turner et al
1995, Birdsay 1996). Estimation of subsequent reductions of carbon
from the decomposing components from the forest module were calculated
using the following equation:
(6) Xt = X0 (1- k * t ) ,
where Xt is the carbon biomass at time t, X0
is the initial biomass, k is the species specific constant
describing the biomass loss per year and t is time in years (Aber
and Melillo 1991). The mass of decomposing material is the sum of
mortality in the most recent interval (5 year periods) and the residual
mass of decomposing material (Xt).
Because LMS projections work on 5 year steps, the equation generally
used within the model follows the form:
Total Xt1 = Xt 0->1 + ((1-k)5 * Xt0),
where Total Xt1 is the cumulative carbon in a certain component at time t1, Xt 0->1 is the carbon accumulated in that component in the period t0 to t1, k is the decomposition rate, 5 is the number of years and Xt0 is the carbon found in that component at time t0.
3.2 The Carbon in Products
Carbon sequestration goes beyond what can be measured in the forest
as live and standing or dead and decomposing. Forest products constitute
a very important pool for capturing carbon on a long-term basis,
especially when emphasizing the use of wood on long term products,
such as lumber for structural components in residential construction.
Products are modeled with a constant rate of products loss to the
atmosphere, as most studies that have addressed products have done
(Houghton et al. 1983, Harmon et al 1990, Oliver at al 1990, Dewar
1991, Harmon et al. 1996). The model does not allow for changes
in time in terms of technological improvements in manufacturing
efficiencies and product use, and does not include disposal since
it includes continuous decomposition. The model considers the raw
biomass harvested, its conversion to products through manufacturing,
and the accumulation and decomposition of the product pool through
time.
The products module takes all the biomass harvested at different
points in time, allocating part of it to long term and part to short
term carbon pools. The long term products constitute the base for
the substitution assessment. The short term products are the base
for the displacement of fossil fuels by biofuels. Harvesting and
manufacturing emissions are also part of the carbon model accounting
(Figure 3).
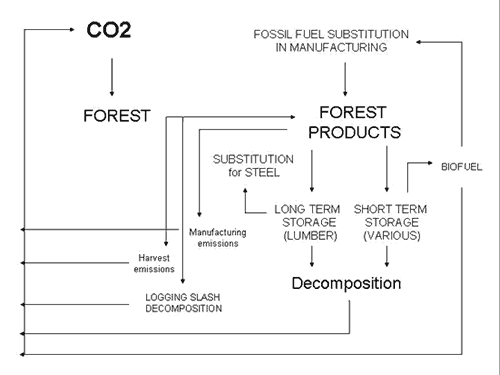 |
| Figure 3. The products module and its components within the carbon model. |
Starting with the Volume summary table from LMS,
with forest and cut volumes for a particular scenario, the amount
of forest products is determined by using a set of studies recently
conducted in the Pacific Northwest by C.O.R.R.I.M. (2002). Four
mills were surveyed in the region, producing dimension lumber as
their primary output. The manufacturing process was divided in four
units: sawing, drying, planing and energy generation. The numbers,
coefficients and factors used in this part of the carbon model are
the average values derived by weighting the production at each one
of these mills (Appendix B).
The spreadsheet starts with total raw volumes of harvested material
per stand given in ft3/acre. Using an average lumber
yield of 9.9 bf/ ft3, volumes in ft3 are converted
to Mbf (thousand board feet) of dry planed lumber. In order to produce
one Mbf of dry planed lumber, 101.01 ft3 of raw logs
are required. This standard yield is neither species nor diameter
sensitive. The four mills reported a range from 90.4 to 105 ft3
of logs /Mbf of lumber. A wood density of 28.08 lbs/ft3
was used for Douglas-fir and 26.21-lbs/ ft3 for western
hemlock (US Forest Products Laboratory, 1999) to convert volumes
to mass.
Co-products from the sawing unit are added to the ones from the
planning unit to give co-product totals for the manufacturing process.
These totals are used as average biomass outputs for co products
based on the volume units (Table 1). The outputs from this part
of the products module are Mbf /volume harvested, the biomass of
dry planed lumber obtained from this volume, an the biomass of co-products,
in English and metric units.
The volume conversion numbers can be modified according to specific
cases, for example, when greater or less efficiencies at the mills
can be accounted for. These conversion numbers were taken from the
average of the operations and efficiencies for the mills surveyed
in the PNW region. They combine an 86% recovery at the planer, with
56% recovery at the sawmill, giving an overall yield of approximately
48% for planed dry lumber from raw logs. At this level of the analysis,
100% of the harvested material was considered lumber yield material,
with no differentiation towards plywood material.
The products carbon content is assumed to be approximately 50% of
the dry weight biomass (Birdsay 1996, Winjum et al 1996). Co-products
are green and hog fuel is assumed to be at 50% wet base moisture
content, a value given by the mills.
The carbon pools of lumber and co products were estimated to decompose
according to species-specific annual decomposition rates (Harmon
et al 1996, Winjum et al 1996). Estimation of carbon loss to the
atmosphere through decomposition were calculated using equation
(6) with specific constants describing the decomposition of long
and short term storage products. The total products carbon at time
1, Xt1, is the sum of products harvested and manufactured
in t0 decomposed for the 5 year interval between t0 and
t1, plus the products harvested and manufactured in t1.
This calculation works for long term and short term storage products,
and the decomposition of these two products pool is calculated separately
within the products module of the carbon model.
3.2.1 Carbon Emissions
Carbon emissions from forest operations are based on the amount of fertilizer, lubricant and fuel consumed in the intermediate operations (pre commercial and commercial thinning, fertilization) and final operations (harvesting). Emissions from regeneration activities are not accounted for because they are not significant (Johnson personal communication. 3/2002). The amount of fuel and diesel consumption depends on the harvesting equipment, the amount of fertilizers applied at the seedling stage and as intermediate operations, the intensity of silvicultural treatments, and the distance to the mills for processing of the logs. Carbon emissions are estimated and defined based on outputs from the SimaPro model (Franklin Assoc. 1998) and Johnson's harvest factors (personal communication, 3/2002) and will be explained later in this part of the chapter.
Diesel consumed is estimated to be 0.0184 gallons/ ft3
of timber volume extracted for the regeneration harvests, and 0.0246
gallons /ft3 of timber volume extracted from the thinning
operations (Keegan et al. 1995). The lubricant consumption is assumed
to be 1.8% of the fuel consumption (Kellog at al. 1996). Diesel
for hauling corresponds to 0.0276 gallons/ ft3 of timber
volume transported (0.0006 gallons/mile of transport). Hauling production
and fuel consumption include empty and loaded travels over the specified
distance. This distance can be changed according to the reality
of the projection. For the case studies the distance is assumed
to be 35 miles one way.
By using conversion factors of 138,881 British Thermal Units (BTU)/
gal for diesel and 148,832 BTU/ gal for lubricants (Johnson 2002),
the amount of BTU/ acre from the use of these two products can be
determined for the amount of volume harvested on the acre and hauled
from the simulated stands at any particular point in time. From
this, the amount of BTU/ft3 can be determined as well.
The harvesting and regeneration emission factors from the SimaPro
model are given in kg of compound emissions/ MegaJoule (MJ) (Table
2).
| Table 2. Emission factors from the SimaPro model. (Franklin Assoc. 1998). | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
Joule is the metric unit for energy. The amount of diesel and lubricant in BTU/ft3 must be converted into metric units. For this, the volume from each silvicultural operation in ft3/ acre is multiplied by 0.7 to obtain m3/ha. With volume and area in metrics, MJ/m3 can be determined. The output in MJ/m3 is multiplied by the SimaPro emission factors (kg/MJ) to obtain emission outputs in unit weight/ unit volume harvested in metric units and are converted to English units (kg/ m3 and from that to lbs/ ft3 ). Emission compounds addressed by this part of the module are: carbon monoxide (CO), fossil carbon dioxide (CO2) and non-fossil carbon dioxide (Franklin Assoc. 1998). Fossil carbon dioxide represents the emissions from fossil fuels, and the non-fossil carbon dioxide comes from the burning of biomass in a wood boiler.
The emission numbers are presented in terms of total carbon emissions in kg/ha and lbs/acre for the volume harvested every time a treatment is performed. The amount of carbon emissions is determined by multiplying the total emissions by the atomic weight of carbon and dividing by the atomic weight of the compounds in question (CO: 12/28 and CO2 : 12/44). Carbon emissions from forest operations are cumulative through time because less than 2 % of the emissions come from non fossil sources.
The amount of fertilizer for regeneration is estimated at 0.0009 lbs of nitrogen, 0.00016 lbs of phosphate and 0.00039 lbs of potassium/ seedling (Schlosser et al, 2001). The intermediate fertilization is assumed around age 30, with 465 kg of urea/ ha (413 lbs of urea/acre) which should leave about 225 kg/ ha (200 lbs/acre) of nitrogen in the ground. Fertilization emissions are based on an average rate of 50 ha/ hour (120 acres/hour) for the amount of fertilizer assumed (Webster, personal interview 11/2002). Using average density of 12.97 barrels per metric ton for liquid petroleum gas (LPG), and 42 gallons per barrel, the weigh of LPG is 1.8 kg/ gallon. With an 80 % of weight in carbon, and 1.67 gallons of LPG/acre required for the running of an average size helicopter used in these types of fertilizations (Johnson 2002), the emissions are estimated to be about 6 kg /ha (5.3 lbs/acre), an almost insignificant addition, nevertheless accounted for in the process.
3.2.1.2 Manufacturing emissions
Manufacturing emissions from the production of one Mbf of planed dry lumber were exported from the CORRIM database (CORRIM 2002 unpubl.) determined by using the SimaPro model (Franklin Assoc. 1998) (Table 3). The Life Cycle Assessment protocol established that manufacturing emissions are to be accounted as burdened emissions. This means the manufacturing emissions shown in Table 3 are only 56% of the sawing emissions and 86 % of the planning emissions, with the co-products burdened with the remain emissions.
| Table 3. Air emissions from production of one MBF dry planed lumber | ||||||||||
|
Unit factors for the carbon emissions were established for the manufacturing process considering a mix of Douglas- fir and western hemlock in the production of dry planed lumber. The ratio of the mix between the two species is not considered to affect the overall air emissions, since the two species are very similar. These unit factors for CO, CO2 fossil and non-fossil, and CH4 are multiplied by the number of Mbf produced from the harvested volumes and summarized in terms of kg/ha and lbs/acre of carbon emissions. Drying emissions are assumed to be 0.009 kg carbon /Mbf (0.2 lb carbon/MBF) as reported by the mills and are part of the overall manufacturing emissions output. Manufacturing emissions are further summarized in metric tons/ha and thousand lbs/acre and are cumulative through time.
All manufacturing emissions in this phase of the model are considered to accumulate through time because the majority of the emissions are fossil emissions. However, this fossil fuel usage is a reflection of the mills surveyed, and not necessarily the reality of the region. In fact, more than 50% of the energy for lumber production comes from biofuel usage according to data gathered recently. This information on the biofuel usage was just recently updated (Wilson, personal communication, 10/2002) and further adjustments within the products module need to be done to better differentiate between fossil and non fossil emissions, the emissions from non-renewable and renewable resources.
| Table 4. Updated air emissions from production of one MBF dry planed lumber. | ||||||||||
|
3.2.2 Displacement of Fossil Fuels by
wood
The amount of carbon stored in the biosphere reaches
a steady state, and continuing mitigation of carbon emissions will
depend on the extent to which fossil fuel use is displaced by biofuel
(wood energy) and the level of wood products usage.
As long as the wood used for fuel in the wood boiler is replaced
by new growth, there is no net increase in the amount of CO2
released. The carbon in the fossil fuel not used, having being displaced
by the usage of wood, remains in storage. This storage is cumulative
through time since the displacement of fossil fuel is permanent
(Burschel et al 1993). Manufacturing emissions are burdened for
the use of the co-products as source of energy with 100% of the
emissions from the manufacturing of lumber together with the co-products
outputs.
These factors, together with numbers taken from the Energy Information Agency (2001) on average carbon emissions from different fossil fuels are the basis for the displacement calculations. The share of co-products that are used for their fuel value varies widely depending upon their price relative to alternative energy sources. Maximum displacement at harvest uses all of the co-products (bark, chips, shavings, sawdust and fuel bark) as biomass for energy (i.e. fuel for the wood boiler). This biomass permanently displaces the fossil fuels that would have been burned to produce the same amount of energy.
With an average of 75% carbon per unit weight, 0.0145 metric tones of carbon are released for every million BTU produced with a natural gas boiler (1000 ft3 = .816 million BTU). With an average of 80% carbon per unit weight, 0.01716 metric tons of carbon are released for every million BTU produced with a diesel boiler.
For the case studies evaluated in the applications of the carbon model, natural gas boiler was evaluated against a wood boiler; therefore the displacement equation follows the form:
Displaced carbon in Metric tons/ha = BTU produced with hog fuel/ ha * 0.0145.
3.2.3. Substitution: wood vs. steel in construction
When managing a fix number of hectares while producing
more or less products through different management scenarios, substitution
by other more energy intensive products will occur when wood products
are not available from the fixed land base.
The substitution analysis assesses the contribution of a single
hectare to the already existing mix of wood and steel framed houses
for a certain region. The region, in this context the Minneapolis
region, has a market in which about 100,000 new houses are build
every year, with 80,000 wood framed dominant and 20,000 steel dominant.
The assumption is that adding one wood house to this matrix results
in the subtraction of one steel house, so that all measurements
are consistent with a fixed unit of consumption, in this case a
fixed number of house units providing equivalent service.
Starting from a base case scenario with a defined wood flow through
time (in this case the 40 Base Rotation), the wood for about 4 wood
framed houses out of the total market comes from our hectare at
harvest. The contribution coming from any change in the management
of our hectare to the change of that mix of wood vs. steel framed
houses is what is being considered by the substitution analysis.
When more wood is produced, less carbon will be emitted per hectare
because less steel will be needed and less fossil fuel will be used.
More carbon is emitted per hectare when wood is not available on
our hectare because steel, a higher energy manufacturing product,
has to be used instead of wood.
The total wood biomass required for a wood framed house is roughly double the wood biomass required in the steel framed design. The biomass harvested, in metric tons/ ha of wood at different periods in time, is the base for the assumption that wood design construction is applied when wood is harvested on the hectare and steel design construction is applied when wood is not harvested on the hectare.
There are two equations used in the calculations for substitution:
1. X t1 (total wood harvested at time t1) = 14.37 Hw + 7.74 Hs
H = number of houses, w = wood, s = steel ,
where the coefficients represent the wood mass in each house (14.37 metric tons for the wood framed house and 7.74 metric tons for the steel framed house).
2. Hw + Hs = 1
Solving two equations for two unknowns, the number of potential wood framed and steel framed houses is determined based on the amount of wood fiber harvested at a particular point in time:
Xt1 (total wood harvested at time t1) = 14.37 Hw + 7.74 (1-Hw)
Xt1 = 6.63 Hw + 7.74 => Hw = (Xt1- 7.74)/ 6.63
=> Hs = (Xt1- 14.37*Hw)/ 7.74
When no wood is harvested in our hectare, Xt = 0 and the calculation establishes that the regional matrix of houses will have 1.17 less wood framed houses and 2.17 more steel framed houses than the base case. Fractional units simply indicate the contribution share for our one hectare example.
The Global Warming Potential (GWP) index (CORRIM 2002, app G) was used for the calculation of the tradeoff in terms of carbon emissions of the construction of a wood framed house versus a steel framed house. The estimates of carbon emissions for both house designs have been calculated from the resource extraction to the construction of the buildings, with all steps in both processes accounted for. The number of houses, Hw and Hs at a particular point in time, are then multiplied by their respective GWP Index to determine the amount of CO2 sequestered and emitted by such constructions. The GWP Index is the impact assessment in terms of CO2 released to the atmosphere, together with the equivalent of other gases with global warming effects. The equation is as follows (EMR Canada 1990):
GWP index (kg) = CO2 (kg) + [3 CO(g) + 150 Nox(g) + 63 CH4 (g)]/1000
The GWP index is 39,810 equivalent CO2 kg for the wood framed house, and 59,290 equivalent CO2 kg for the steel framed house. These numbers are the base for the CO2 emission substitution equation that has the following form:
CO2 tradeoff equivalence = - Hw * 39,810 + Hs * 59,290.
This number is later converted to carbon emissions
by multiplying it by the atomic weight of carbon with regards to
the CO2 molecule. If more wood is harvested at any point
in time in our hectare, more wood framed houses will be built with
contributions coming from our hectare, representing fewer carbon
emissions per hectare.
The substitution numbers are the result of deducting the differential
in carbon emissions from an alternative management scenario with
respect to the 40 Base Rotation. Because the substitution numbers
come from the differential of the Base case to an alternative management
scenario, if no wood is harvested in any of the hectares considered,
there is no difference, they cancel each other out. The differences
only arise when wood is harvested in one scenario and not in the
other one.
4. RESULTS
The alternatives considered characterize the effects of changing
the rotation lengths (the Rotation case) and alternative management
intensities (the Intensity case). A base case scenario is selected
as a reference for the analysis. The rotation analysis compares
the base case to longer rotations, over different periods of time.
The study evaluates carbon pools at the forest and products levels,
and considers trade offs in terms of carbon displacement (fossil
fuel displacement by wood burnt for energy) and substitution in
construction with wood versus steel. The Intensity case assesses
the effects of more intensive management, and trade offs of slightly
longer rotations (10 years) combined with higher management intensities.
The Intensity case, like the Rotation case, provides a detailed
description of the carbon pools and its differences in the forest,
in products, with displacement and substitution impacts. Finally,
the economic implications for these different cases are provided.
4.1 The Rotation Case
The afforestation case is selected as a starting point because
of its tutorial properties and its simplicity. Afforestation implies
the establishment of a new forest on non-forested land. A typical
example of afforestation is to grow trees on degraded agricultural
or pasture land. Afforestation implies a one time increase in the
sink capacity of a certain area, and after the first rotation has
been established and harvested, the development of carbon pools
is similar to a reforestation case.
The base case is selected using LMS and stand inventory data. The
FVS growth trajectory was matched to the McArdle et al.(1949) yield
table for similar stands. In order to create the inventory used
in the rotation and management intensity case scenarios. A newly
created plantation with a base site index of 128 was matched to
the number of trees per acre, the quadratic mean diameter, the height
and volume of a 20 year old stand from McArdle (1949). The FVS PNW
variant was then adjusted to track the change in yield shown in
the McArdle table, by adjusting the maximum stand density index
and growth rates. With the growth model calibrated in such a way,
stands with the same inventory but with different site indexes were
created. An average site index of 110 was picked for the base case
simulation. All of the simulations had a pre commercial thinning
at age 15 and a regeneration harvest at different points in time:
40, 45 and 50 year rotations.
The three rotations were later evaluated for economic optimization.
Zobrist's economic model for the Pacific Northwest region was used
(2001). It provides Soil Expectation Values (SEV) for the scenarios
in question. SEV is a forestry term used to describe "the present
net worth of bare forestland for timber production calculated over
a perpetual series of timber crops grown on that land" (Davis
and Johnson 1986). Maximizing SEV is the correct way of guiding
an investment, and the management decisions to achieve economic
efficiency. The economic model includes management costs and a set
of other variables that must be specified (Appendix D). Assuming
an interest rate of 5%, the 40 year rotation produced the highest
SEV at $463. The forty year rotation therefore was selected as the
base case scenario for both the Rotation and Intensity case scenarios.
The Base 40 year scenario is pre commercially thinned at age 15 to 680 trees per hectare (275 TPA). The 80 year scenario is pre commercially thinned to 680 trees per hectare, and commercially thinned twice, leaving about 2/3 of the total basal area at ages 30 and 60. The 120 year scenario is the same as the 80 year scenario prescription, with one extra commercial thin at age 90. The No Action scenario has nothing done to it and assumes no natural disturbances beyond the normal mortality. All scenarios start with 1900 trees per hectare (770 TPA), and the same number of trees is planted after every regeneration harvest (2.1* 2.4 meter spacing). Regeneration harvest under these scenarios is equivalent to zero retention at time of harvest. The No Action scenario is not equivalent to what one would expect in natural stands since they would generally have had poor stocking and contain many fewer trees per hectare.
Forest carbon results will be presented individually by scenario. Results for the carbon in products, emissions, displacement and substitution will be summarized and compared for the four scenarios combined.
4.1.1 The Forest Carbon
The standing carbon found on site increases from zero in year 2000 to a total of 154 metric tons/ha after the first rotation. By the end of the second rotation, the standing total increases to 182 metric tons/ ha, reaches 191 by the end of the third rotation, and 196 metric tons/ha by the end of the fourth one. Total forest carbon is the amount of carbon found on live and dead components in the last year of the rotation, just prior to the regeneration harvest (Table 5). Trees used for the reforestation simulations (years 2040, 2080 and 2120) are older than the ones planted in the afforestation (2000), therefore slight differences in canopy, stem and live roots can be appreciated in the forest carbon.
| Table 5. Forest carbon in metric tons/ ha prior to final harvest through time. | ||||||||||||||||||||||||||||||||||||||||
|
When compared to the total forest carbon in year 2040, there is an increase of 18% in the total forest carbon at the end of the second rotation, a 24% increase by the end of the third rotation, and a 27% increase by the end of the fourth. The carbon pools are asymptotically approaching a steady state: the reforestation condition starting from the case of afforestation. The carbon in the canopy, stems, snags and live roots reaches this stability more rapidly than the carbon in the dead roots and litter.
The tree stem represents about 68% of the total forest carbon after the first rotation, and because of the increase in the litter and dead root components through time, this percentage decreases to about 60% in the following rotation simulations. It is the increase in the litter and dead root pools that contribute the most to the increase of carbon pools on site.
The numbers from Figure 4 for the forest carbon pools do not correspond to the numbers found in Table 4, since the figure shows the stand after having been harvested in years 40, 80, 120 and 160. This explains the increase in the litter pool and the transfer of the live root carbon pool to the dead root in those particular years. The litter and root pools will eventually reach a stable level in time, as more rotations are grown and harvested.
Soil carbon was not modeled. The way this carbon pool will be affected from the case of afforestation to reforestation, as stated in the Literature review chapter, will mostly depend on the method of harvest and the site preparation activities for the regeneration planting. However, for this evaluation it is assumed that it should have little consequence in the comparisons where the focus is on modest changes between rotation lengths.
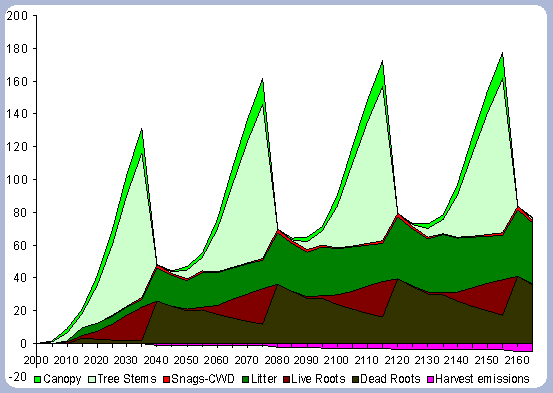 |
| Figure 4. Forest carbon pools in metric
tons/ha for the 40 year scenario (after harvests in 2040, 80, 120, 160). |
The forest carbon reaches 228 metric tons/ ha after the second rotation, an increase of 4.5% from the first 80 year rotation (Table 6). The small percentage increase of the forest carbon pool is mainly due to the decomposition of dead roots and litter, with decomposition rates of 5 and 16% per year respectively. The stem, canopy and live roots pools remain relatively constant, when looking at the end rotation results by the end of the second rotation.
| Table 6. Forest carbon in metric tons/ ha prior to harvest through time | ||||||||||||||||||||||||||||||||
|
The increase in the forest carbon pool is due mainly to the increase
in the snag, dead root and litter component (Figure 5). The canopy
carbon pool reaches about 16 metric tons/ ha at age 50 and will
stay relatively constant after that age until harvesting time. The
litter pool increases to 25 metric tons/ha after the first rotation,
and reaches 33 metric tons/ ha after the second rotation. All the
live root carbon is transferred to the dead root pool after harvest,
reaching 44.15 metric tons/ ha after the first rotation and almost
50 metric tons/ ha after the second. It is expected that the litter
and roots carbon pool will reach a stable asymptotic level after
the stand has been carried under this management strategy for several
more rotations.
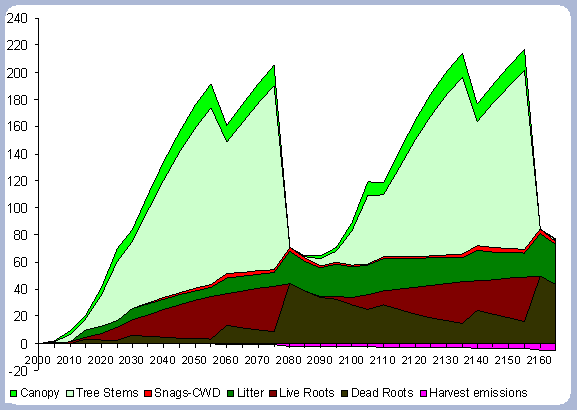 |
| Figure 5. Forest carbon on two 80 year rotation in metric tons/ha (after Harvest). |
4.1.1.3. The 120 year rotation and No Action scenarios
These two scenarios will be evaluated together in terms of their forest carbon pools. In terms of treatments, the 120 year scenario has had a PCT early in the rotation and three commercial thinnings, therefore the forest biomass and carbon pools will be significantly different. The No Action scenario should not be confused with natural stands, which are generally characterized by poor stocking and periodic disturbances within the first 120 years. The No Action scenario would be subject to a higher risk of disturbances, especially fire and diseases associated with overly dense stands.
There is 13% more carbon in the forest pools of the
No Action scenario than in the 120 year scenario, most of it accumulated
in the stem and live roots, with a difference of 20 and 18% respectively
in those particular components between the two scenarios (Table
7).
| Table 7. Forest carbon pools in metric tons/ ha prior to harvest in 2120, compared to a No Action scenario | ||||||||||||||||||||||||||||||||
|
Not forgotten must be the fact that the 120 year rotation
was commercially thinned three times through the scenario (ages
30, 60, 90): The No Action scenario is above the 120 year scenario
at all points in time due to this fact. The No Action scenario also
has more carbon in snags, which implies the effectiveness and higher
productivities achieved when treatments to reduce competition are
applied (Figure 6).
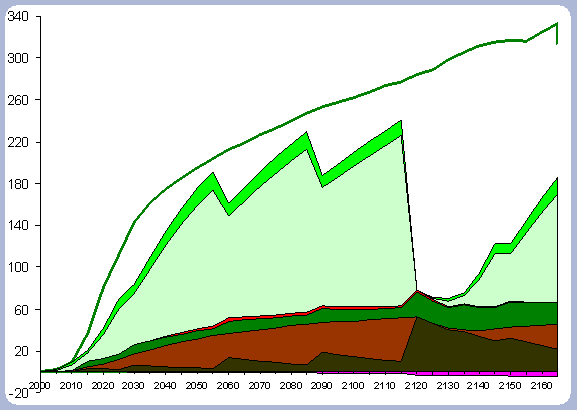 |
| Figure 6. Forest carbon pools (after harvest in 2120) for the 120 year rotation scenario vs. No Action management scenario (No Action total forest carbon = top line). |
Carbon sequestered in the live roots amount to about
18% of the total carbon in the forest pools in the 120 year scenario,
versus 19.5% of total carbon in live roots pools for the No Action
scenario. There are higher levels of carbon in the litter and dead
root pools in the 120 year rotation because of the different silvicultural
regime. This differentiation starts at the time of the PCT, where
the growth rate for the No Action scenario is still very high. Again,
most of the difference is accounted for by the commercial thinnings
and the differential in the amount of trees per hectare. The No
Action scenario does not seem to have been slowed down by competition,
and mortality seems very low when looking at the total carbon in
the snag component and comparing that number to the snags found
in the 120 year rotation, which is about half, but had four intermediate
operations (PCT and 3 CT).
On a pure forest carbon basis, the No Action scenario forest pools
are greater than any of the other scenarios as a consequence of
the lower removals and higher overall stocking through the management
period considered.
4.1.1.4. Summary forest carbon with different rotations
A summary for the carbon stored in the forest is provided
on a net basis, meaning the carbon emissions coming from silvicultural
operations are calculated and deducted from the total carbon pools
(refer to Methods section for emission calculations). Thus, net
carbon sequestered through the different scenarios is the sum of
the carbon in all live and dead pools through the management period,
with harvest and thinning emissions subtracted.
When considering carbon sequestered in the forest while accounting
for the emissions from operations on site, the No Action scenario
is above any other scenario. The 120 year scenario follows, and
is slightly higher than the 80 year scenario. Last in this list
is the shorter 40 year rotation.
The residual of carbon carried on from one rotation to the next
in the litter, snag and root pools increases from 50 metric tons/ha
to about 70 metric tons/ha in 165 years for all the rotations in
which management comes into place. The results for the increase
in the litter pool assume that no fire is used as a site preparation
tool. Fire would volatilize much of the carbon that is considered
sequestered after the harvest (Figure 7).
Carbon pools are estimated every five years, because there is not
a continuous measure of the inventory in between the five year management
steps simulated. In order to come up with estimates of the areas
under the different curves, the total carbon in the forest pools
at the beginning of each 5-year management step is multiplied times
five and then added together.
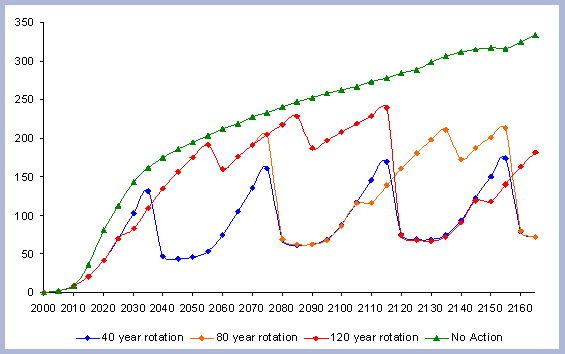 |
| Figure 7. Net Carbon in
the forest pools for all rotations through time in metric tons
/ha, with emissions from operations deducted. |
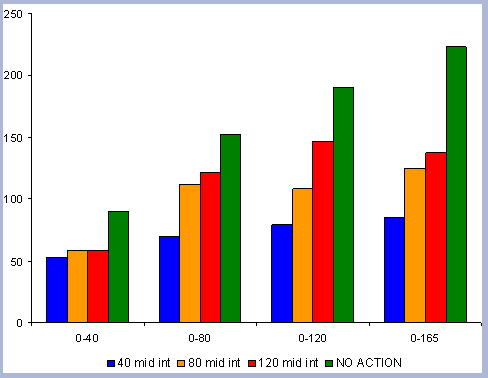
From these calculations, average annual carbon sequestered
in the forest can be estimated for various intervals of time (Figure
8). Periods encompass forty years and do not consider the last harvest
at year 40, 80, 120 or 160 to have been materialized. The Average
carbon in the forest pools is the highest for the No Action scenario,
for any interval of time considered.
 |
| Figure 8. Average carbon in the forest pool for all rotations at different intervals of time in metric tons/ha with emissions from operations deducted |
Over the total period of 165 years, the No Action
scenario supports an average of 223 metric tons/ ha. As a comparison
and reference, the median old growth volume as a single point estimate
is 11012 ft3/ acre. The volume
number was derived from the FIA riparian data and the Prime database
using all unmanaged stands over 80 years in the sample (Woundenberg
& Farrenkopf 1995). The median old growth volume corresponds
to the volume found in year 2110 for the No Action scenario. This
is to show the substantial potential differences between Old Growth
and the No Action afforestation case, where on average, according
to the volume estimate, and old growth stand would support about
200 metric tons/ ha on average over the same period of time.
At the other extreme is the base, the 40 year rotation, which when
considering the 165 years of management sequesters an average of
85 metric tons/ha. Over this interval, the No Action scenario provides
163% more carbon than the 40 year rotation; the 80 year rotation
provides 47% more and the 120 year rotation 62% more carbon. These
averages change somewhat depending the time interval considered.
For example, during the first 80 years of management, the average
carbon in the forest pools for the 40 year scenario is about 3/5
the carbon in the 80 year scenario, because the 80 year rotation
has not been harvested yet. The 120 year rotation is about twice
on average the carbon in the 40 year rotation when the interval
in question is the first 120 years of management, and harvesting
has not yet occurred.
4.1.2 The Carbon in Products
Carbon sequestered in wood products is an important pool, and
its importance and relevance to the carbon story will depend on
the permanence and useful life of those products. On a 165 year
management scenario, there are four regeneration harvests done on
the 40 year rotation, two on the 80 year rotation and a single on
the 120 year rotation. Volumes harvested at the end of the rotations
ranged from 5210.68 ft3/acre for
the shorter rotation to 9562.76 ft3/acre
for the longer rotation. Totals harvested, including commercial
thinnings, increased this number to 15359.07 ft3/acre
for the 120 year rotation, 10935.23 ft3/acre
for the 80 year rotation. Since the 40 year rotation only had a
pre commercial thinning, the total volume harvested stays the same
(Table 8).
| Table 8. Volumes from commercial thinning (CT) and final harvests for different rotation lengths in ft3/ acre. | ||||||||||||||||||||||||||||||
|
In terms of total volumes harvested, the numbers are
almost equal when considering time. The volume from a single 120
year rotation, 15359 ft3/acre,
is about the same as three 40 year rotations, totaling 15632 ft3/acre.
On the same track, a single 80 year rotation, 10935.23 ft3/acre,
is about the same as two 40 year rotations, 10421.36
ft3/ acre.
The volumes harvested are converted to biomass by using a standard
wood density of 28.08 lbs / ft3
for Douglas-fir, and later converted to metric units to be consistent
with metric tons/ ha units. The repartition of the biomass on a
dry weight basis into different products and co-products in kg/
MBF of planed dry lumber is shown in Table 9 (derived from Table
1.4, App B, CORRIM 2002).
Approximately half of the biomass harvested goes to lumber, with
the other half distributed among products with much shorter useful
lives. Once the biomass is defined, it is turned into carbon pools
using percent carbon ratios for the species (Birdsey 1992).
| Table 9. Biomass distribution in kg of forests products/MBF of planed dry lumber produced. | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
The longevity of lumber against shorter lasting products is the reason why the close to a half ratio between long and short term products in terms of biomass and carbon is not consistent with the longer rotation numbers (Table 10). The 80 and 120 year rotations have had 2 and 3 commercial thinnings respectively through the rotation, which increases the longer lasting products pool against the shorter pool that readily decomposes.
| Table 10. Total products in metric tons of carbon /ha for all rotations. | ||||||||||||||||||||||||||||||||||||||||
|
There are 49.7 metric tons/ha of carbon in lumber
on the 40 year rotation. The 80 year rotation, through harvest and
commercial thinnings, is 200% the carbon from the lumber manufactured
in the base case, at 100.3 metric tons/ha. The 120 year rotation
is 260% the carbon in lumber and 194% the carbon in short term products
from the 40 year rotation. For the short term products carbon pools,
the 80 year rotation is about 164% the amount sequestered by the
40 year rotation. No Action produces no products.
The volume trend shown in Table 9 differs from the carbon trend
because of decomposition factors that are incorporated in the carbon
analysis but not in the volume totals given by rotation. The carbon
pools established in Table 7 are totals at the end of each rotation.
The commercial thinnings for the 80 year rotation take place at
years 30 and 60, which means the long and short term products obtained
from those operations (about 40% of the total volume of the rotation)
have been decomposed for 50 and 20 years respectively. The same
is true for the 120 year rotation, which thinnings at ages 30, 60
and 90, encompass about 38% of the total volume from the rotation,
and have been decomposed for 90, 60 and 30 years respectively.
The decomposition equation is taken from Aber and Melillo (1991)(See
Methods section), and decomposition rates are 1%/year for lumber
and 10%/ year as average for the short term products pool. The product
pools are decomposed using a simple decay model which compared to
a fixed life decay model would have a different impact on the thinning
treatments and short rotation scenarios.
4.1.2.1 Carbon in Long Term Products: lumber
There are four harvests within the same period of time for the
40 year rotation compared to a single one for the 120 year rotation,
therefore an analysis through time is pertinent to the overall understanding
of product's trade offs in the carbon story (Figure 9 and 10).
The lumber is decomposed through time at a 1% per year. By the time
of the fourth 40 year rotation regeneration harvest, the cumulative
amount of carbon sequestered in lumber products is highest for the
40 year rotation, followed by the 80 and 120 year rotations. The
average carbon sequestered in the lumber pool through the management
scenario is highest for the 40 year rotation, with 64 metric tons/
ha followed by the 80 year rotation with 58 metric tons/ ha, or
90%. On an average basis, the 120 year rotation sequesters in the
lumber pool about 80% of what the 40 year rotation does (Table 10).
A bias exists when considering any specific interval of management
for calculating the averages, therefore different intervals of time
need to be addressed.
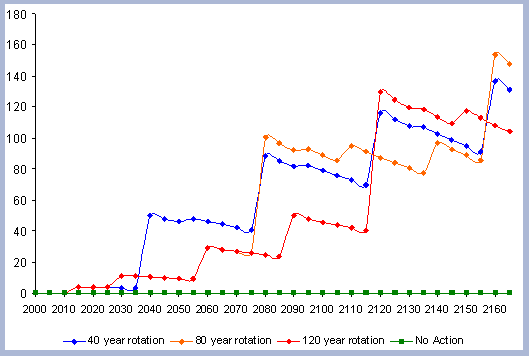 |
| Figure 9. Long term products
carbon pool in metric tons/ha of carbon for all scenarios through the 165 years of management. |
4.1.2.2 Carbon in Short Term Products
The short term products pool decomposes at an average rate of
10% per year, and essentially disappears before the next regeneration
harvest for all the scenarios (Figure 10).
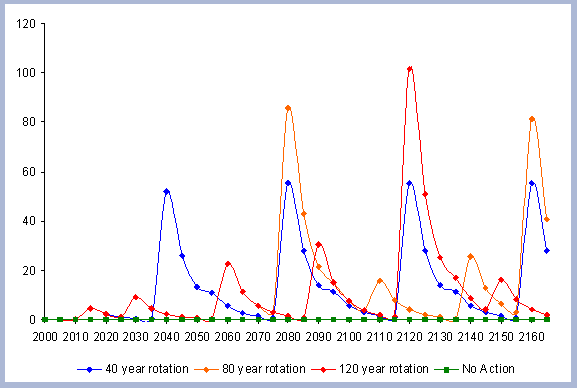 |
| Figure 10. Short term products
in metric tons/ha of carbon for all scenarios through the management cycle. |
The average carbon sequestered in the short term products pool is the highest again for the 40 year rotation, followed by the 80 and the 120 year rotations (Table 11). The total amount of carbon sequestered in the short term products pool is about 20% of the lumber pool for all rotations. This percentage will increase if the number of years the material has been decomposed decreases (i.e. for the 120 year rotation, the number is 22%). The No Action scenario sequesters nothing in products since no harvesting operations are conducted.
| Table 11. Average carbon sequestered in products pool through the 165 year management cycle in metric tons /ha compared to the 40 year base. | ||||||||||||||||||||||||||||||||||||||||
|
The estimated amounts of carbon sequestered in the products pools presented above are not net estimates because they do not include emissions from harvesting and manufacturing activities. The emissions are cumulative from rotation to rotation and must be deducted from the total amount of carbon sequestered to make the analysis consistent.
4.1.2.3. Carbon emissions from Forest operations and Product Manufacturing
Forest operations and manufacturing emissions are shown as negative
values because they are conceptually the opposite of sequestration
(Figure 11). By the time of the fourth regeneration harvest for
the 40 year rotation, the 80 year rotation has accumulated 86% of
the base case harvesting emissions, and the 120 year rotation about
71%, with periodic averages of 2.1 metric tons/ha for the 40 year
scenario, 1.8 for the 80 year scenario and 1.5 metric tons/ha for
the 120 year scenario through the 165 years of management evaluated
(Table 12).
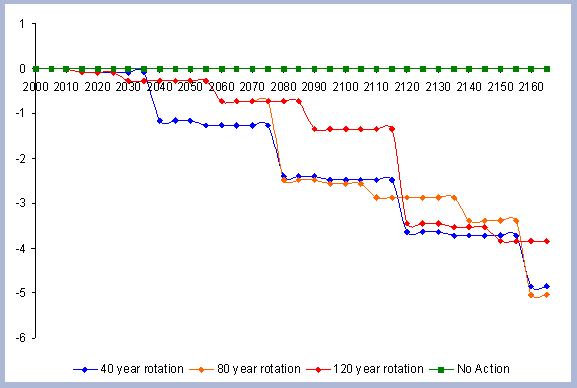 |
| Figure 11. Forest operations emissions in metric tons of carbon/ha for all scenarios through the 165 year management cycle. |
The manufacturing emissions, as expected, are also the highest for the 40 year rotation, since there are more activities prescribed through time. Manufacturing emissions are about ten times the harvesting emissions (Table 12). Periodic average manufacturing emissions for the 165 years considered, are 23 metric tons/ha for the 40 year scenario, 20 for the 80 year scenario (87% of base) and 17 metric tons/ha for the 120 year scenario (74% of base) (Figure 12, Table 12).
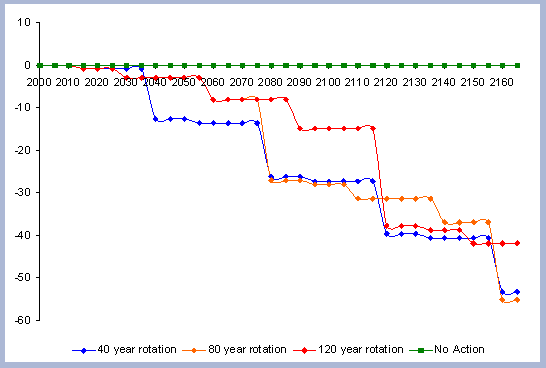 |
| Figure 12. Manufacturing
emissions in metric tons of carbon/ha for all scenarios through
the 165 year management cycle. |
Average total emissions through the 165 year management cycle are 25 metric tons/ha for the 40 year scenario, 22 metric tons/ha for the 80 year scenario (88% of base) and 18 metric tons/ ha for the 120 year scenario (72% of base). The No Action scenario produces no emissions.
| Table 12. Average harvesting and manufacturing emissions for all rotation scenarios in metric tons/ha for 165 years of management. | ||||||||||||||||||||||||||||
|
We can now calculate net carbon in products by deducting the emissions from the operations throughout the scenarios.
4.1.2.4. Average net carbon in Product pools
Net product pools are the accumulation of the products pools with
emissions from forest operations and manufacturing subtracted. Figure
13 shows net product pools for all rotations through the 165 years
of management. The net carbon in products pools increases with each
regeneration harvest, for all rotations.
The 40 year rotation sequesters more carbon than any of the other
rotations, at any length of interval considered, with a net average
of 53 metric tons/ha through for the 165 year management cycle.
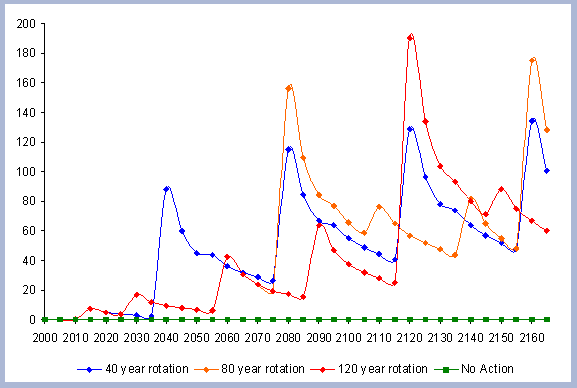 |
| Figure 13. Net carbon in product pools in metric tons/ha for all scenarios (area under curves). |
The 53 metric tons/ha for the base compared to 50 for the 80 year scenario (94% of base) and 43 metric tons/ ha for the 120 year rotation (81% of base), and zero for the No Action scenario (Figure 14, last interval). Periods encompass forty years and do not consider the final harvests at years 40, 80, 120 or 160 to have been materialized. The difference in average net carbon sequestration decreases with increasing years considered, because the longer intervals include more harvesting activities from the longer rotations. It is also important to note that the longer term rotations essentially differ in the entry of products. Since the volume flow into products are different for each scenario, it will be important to understand the consequences of product substitution from any shortfall in wood products.
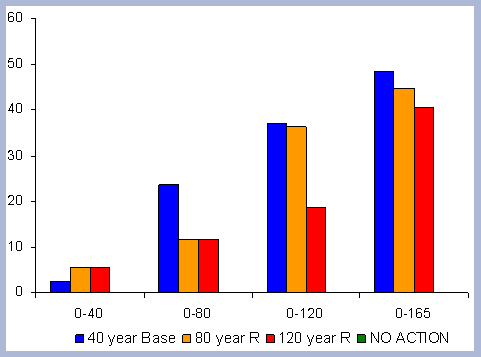 |
| Figure14. Average net carbon sequestered in products pools at different intervals through the 165 year management cycle, in metric tons /ha. |
4.1.3. The Carbon in Forest and Product Pools
The next step in the analysis of the carbon story is to evaluate
the forest carbon together with the net products carbon for all
the different scenarios through time (Table 13). The base case scenario
sequesters the least carbon on average when evaluating the forest
and products pools together, except when considering the first 40-year
interval, where the average for the base is slightly higher than
the 80 and 120 year rotations.
| Table 13. Summary table: Net carbon averages in forest and products pools for different intervals of time through the management cycle in metric tons/ha for all scenarios. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Forest carbon increases with longer rotations and longer intervals while forest products carbon decreases. The No Action scenario forest carbon for the 165 year interval is 60% higher than the base, with forest and products considered. The 120 year rotation is 30% higher and the 80 year rotation is 27% higher (Figures 15 and 16).
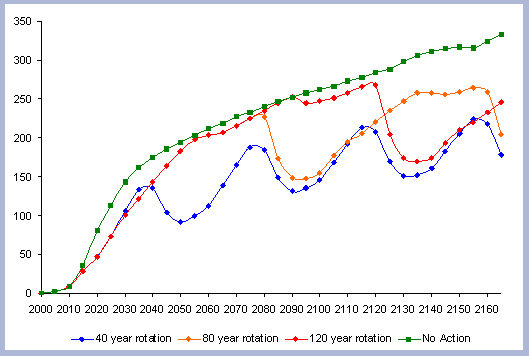 |
| Figure 15. Net carbon sequestered in the forest and products pools in metric tons / ha for all scenarios through the management cycle (area under the curves). |
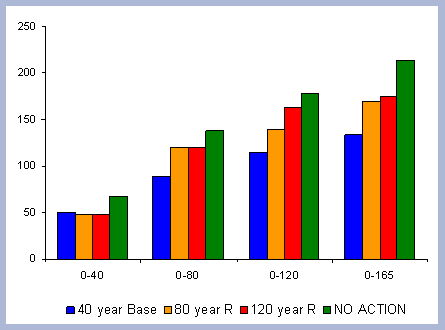 |
| Figure 16. Net average carbon sequestered in the forest and products pools at different intervals of time within the 165 years of management in metric tons/ha. |
The cumulative net products pool for the 40 year rotation reduces the total carbon losses for the short rotation over time but total carbon is still lower than any of the other rotations through the 165 year period (Figure 15 vs. Figure 7 and Figure 16 vs. Figure 8).
4.1.4. Carbon displacement
Carbon displacement, the carbon not emitted from fossil fuels
when wood is used to produce energy, although conceptually important,
does not change the numbers and generals patterns observed previously
among rotations, due to efficiency differences between wood and
fossil fuel burners.
The displacement of fossil fuel based energy by bioenergy increases
with time because the displacement of fossil fuels by renewable
resources is permanent and cumulative through time. Furthermore,
displacement is equivalent to avoided emissions, therefore considered
sequestration in the spectrum of this analysis.
The percentage of short lived co-products that are used for energy
will vary with the economic allocations of those products relative
to their value as energy. In some cases most of the co-products
volume will be used as energy and in other cases very little. Co-products
not used as energy are generally allocated their share of the harvest
and manufacturing emissions burden.
For this simulation and evaluation of the carbon displacement, the
maximum possible displacement impact is developed by burning all
of the co-products for their energy value in a wood boiler. This
is an extreme case, to consider all short term products as hog fuel,
since some of these products have a more valuable usage, such as
engineered wood. The manufacturing emissions are burdened for the
usage of the short term products as energy (see Methods).
The 40 year rotation displaces 38 metric tons/ha per year of carbon
by using less fossil fuels for energy, the highest of the scenarios.
It is followed by the 80 and 120 year scenarios, with averages of
33 and 28 metric tons/ ha respectively (87 and 74% of the base respectively).
The No Action scenario displacement is zero since no harvesting
has occurred (Figure 17).
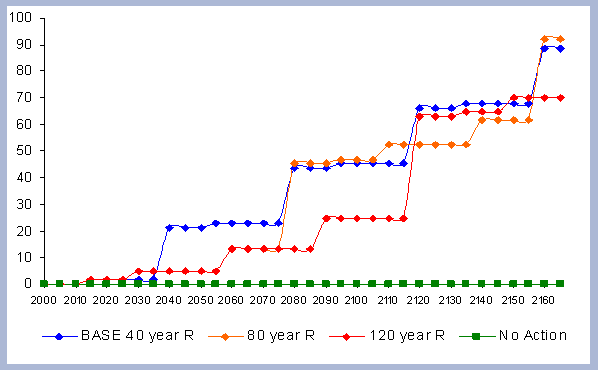 |
| Figure 17. Carbon displacement from fossil fuel based energy by bioenergy in metric tons/ha through time using all of the short term products as hog fuel. |
When incorporating the carbon displacement into the overall carbon story, the benefits of using wood instead of fossil fuels can only be appreciated when longer intervals of time are considered (above 120 years)(Table 14).
| Table 14. Summary Table: Average carbon in forest, products and displacement for scenarios intervals through the 165 years of management in metric tons/ha. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Displacement is equivalent to long term storage and accumulated
from rotation to rotation. The lower levels of efficiency in wood
boilers when compared to fossil fuel burners reduces the displacement
impact compared to long lived products but does not decay like long
live products. It takes a number of rotations to accumulate in displacement
more than the carbon accumulated on average in the short term products.
The relative comparisons between rotations however, are not substantially
different when evaluating displacement together with the other carbon
pools, with the No Action scenario still sequestering more carbon
than any of the other scenarios where harvestings have been done,
in the interval of times considered.
When considering the entire 165 years of management, the carbon
in the No action scenario is about 47% higher than the base, the
40 year rotation, compared to a 60% higher when displacement was
not considered. Displacement is a significant positive contributing
factor if enough time is considered, in this particular case, about
120 years of management are needed to observe its benefits in terms
of carbon displacement.
The average carbon sequestered by the 40 year rotation when considering
displacement in the long term of the 165 year management considered,
is about 10% higher than when displacement does not take place (Figure
18 vs. Fig 15, Table 14).
For the 80 year rotation it is slightly higher than % more and for
the 120 year rotation about 4%. However, the base case still sequesters
less carbon on average than the other rotations. The differences
are smaller, with the differentials in terms of average carbon sequestration
among rotations, decreasing as the analysis moves from the forest,
to the products, to the potential displacement.
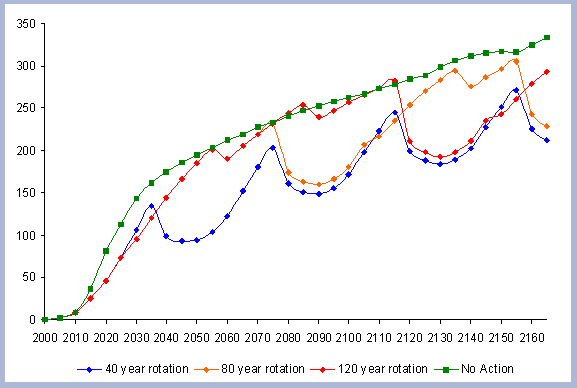 |
| Figure 18. Net forest, products and displacement carbon pools for all scenarios in metric tons/ha accumulating through the 165-year management period. |
4.1.5. Carbon substitution
The environmental analysis framework adopted by CORRIM (2002)
characterizes all inputs and outputs associated with a residential
building unit. Since the management alternatives consider raw material
production changes, substitution of materials is implied when managing
a fix number of hectares through different rotations while producing
the same type of end products.
Number of potential wood framed houses from harvested volumes per
hectare ranges from 4 for the first 40 year rotation, which means
-3 steel framed houses, to up to 9 wood framed houses for the 120
year rotation scenario, implying -8 steel houses built, with the
differential in carbon emissions associated with this different
type of construction (Table 15).
| Table 15. Changes in the number of steel and wood framed houses resulting from harvesting activities or lack of through time on a per hectare basis. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The net forest and products carbon sequestered in the base (40
year rotation) through the 165 years considered represents the baseline
for the substitution analysis. The blue line in Figure 19 is this
base line. Any shortage of wood provided in the other scenarios
result in steel houses substituted with the carbon emissions then
deducted from the previously derived carbon storage in forest and
product pools (Figure 19).
The carbon story is much different when addressing issues pertinent
to substitution. In 2040, the first harvest takes place in the base
case scenario, and since there is no wood harvested in any of the
other scenarios, steel displaces wood at that particular point in
time for those scenarios. The 80 year rotation slightly surpasses
the base case in year 2080, when both the 40 and the 80 year rotation
are harvested, and it will stay higher until the 40 year rotation
goes through its third harvest.
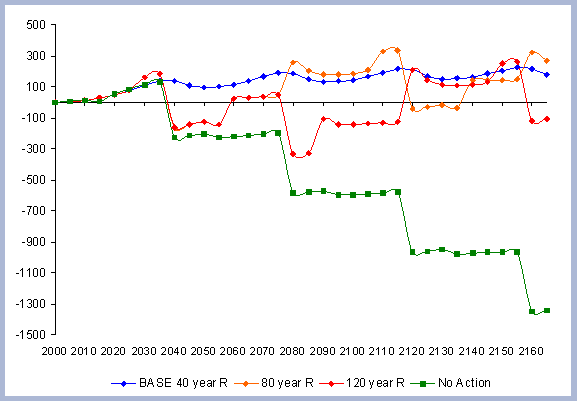 |
| Figure 19. Net carbon substitution for all scenarios through the 165- year management period against the Base 40-year rotation, in metric tons/ ha of carbon sequestered showing trade offs between steel and wood design construction. |
There is a thinning in 2110 that brings the 80 year scenario even
further above the 40 year rotation base case, but this trend is
reversed at the time of the third 40 rotation harvest in 2120. Throughout
this time, because there has been no management in the No Action
scenario, the substitution effect makes the emissions go higher,
reaching 1400 metric tons/ ha of emissions by the end of the management
period of 165 years.
The substitution trade offs are readily observed when the base case,
the 40 year scenario, is normalized to zero (Figure 20).
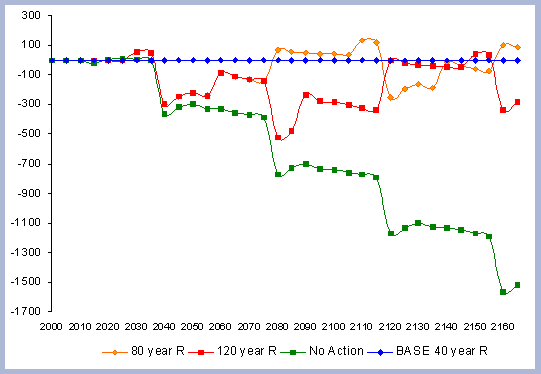 |
| Figure 20. Net carbon substitution for all scenarios through the 165- year management period against the Base 40-year rotation normalized to zero, in metric tons/ ha of carbon sequestered showing trade offs between steel and wood design construction. |
The negative numbers in the scenarios shown below illustrate the
effect of allowing fossil intensive products to substitute for wood
in construction. Over a long period of time, the longer rotations
reduce the rate of substitution ultimately producing a surplus of
wood products somewhere beyond 200 years. The No action scenario
emissions are higher because the benefit of high forest carbon is
more than offset by the amount of fossil fuel energy needed for
construction with steel when wood is not available. The No Action
scenario is followed by the 120 year scenario, for which thinnings
and final harvest in year 2120 are not enough to maintain positive
levels of sequestration. The 80 year scenario is the closest to
the 40 year base scenario, although still sequestering less carbon
for any interval considered within the 165 years.
Looking at average carbon sequestration numbers once the substitution
factor has been incorporated into the carbon story changes the patterns
dramatically, effectively reversing it (Table 16).
With the substitution, the shortest rotation produces the highest
average levels of sequestration for all intervals, ranging from
67 to 140 metric tons/ha on average, depending upon the interval.
The 80 year rotation is sequestering carbon for every interval considered,
although much less than when substitution trade offs are left out.
The 80 year rotation, on average, sequesters half the amount of
carbon through the 165 years considered when addressing substitution.
In this same interval of time, the 80 year rotation sequesters on
average about 40% less carbon than the base case.
| Table 16. Summary table: Average carbon sequestration in forest, products and substitution for all scenarios through the management period in metric tons/ha. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The 120 year scenario on average allows fossil fuels to emit almost 7 metric tons/ha through the 165 years of management. The No Action scenario difference between what it sequesters in the forest and the fossil fuels used instead of wood is equivalent to emitting 496 metric tons/ha on average through the 165 years considered, compared to 223.43 metric tons/ha of carbon sequestered on average through the same period of time when only considering the forest carbon (Figure 21).
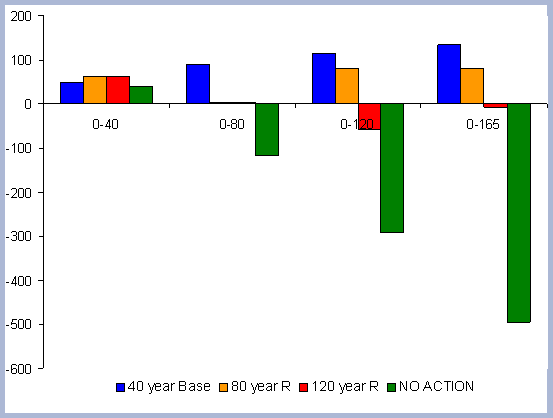 |
| Figure 21. Average periodical sequestration for all scenarios at different intervals in metric tons /ha. |
The No Action scenario sequesters the most carbon when only the carbon in the forest, products and displacement are considered, but contributes substantial emissions once substitution is included in the analysis. The 40 year rotation produced the lowest levels of forest carbon sequestration but with the addition of products and substitution it produces the least emissions, reflected in greater sequestration.
4.1.6. The Economics of the Rotation case
Soil expectation value (SEV) is calculated for the rotations previously
discussed. SEV is a forestry term used to describe "the present
net worth of bare forestland for timber production calculated over
a perpetual series of timber crops grown on that land" (Davis
and Johnson 1986). Maximizing SEV is a means to guide an investment,
by identifying the management alternative with the greatest economic
benefit.
All tree costs are included in the analysis, the guiding interest
rate reflects the context of the Pacific Northwest, and the prescription
for each scenario will be repeated over each rotation. The SEV calculations
are based on Faustmann (1849). Results from calculations of SEV
for the scenarios evaluated previously are presented in Table 17.
Cost assumptions for these calculations are presented on Table 18.
Depending upon the owner's objectives, the return might not be high
enough to allow for the increased risks of disease or other disturbances
that may impact longer rotation scenarios. The 40 year rotation
has the highest SEV therefore if the sole objective was to maximize
the economic return, this would be the scenario to follow.
| Table 17. SEV for all rotations following assumptions cited in Table 18. | ||||||||
|
| Table 18. Assumptions and costs for the calculation of SEV in the PNW rotation case analysis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4.2. Management Intensity Alternatives
The base case for the management intensity analysis is the same
40 year scenario used for the Rotation Case. A total of 3 scenarios
encompassing two prescriptions and two rotation lengths are compared.
The goal is to evaluate trade offs in carbon build up and economics
associated with changes in management intensity. In particular,
does increased thinning and fertilization store more carbon with
what economic impact? And does adding ten more years of growth sequester
more carbon and with what economic effect?
There are two stands and two intensities of management in question.
Both stands are planted in year 2000 with 1900 Douglas-fir trees
per hectare (770 TPA). The base case stand has a site index of 110
(King, 1966), and the only silvicultural treatment is a pre commercial
thinning at age 15 to 680 trees per hectare (275 TPA).
The high intensity management stands are assumed to be fertilized
twice, at age 20 and 30. The silvicultural treatments associated
with the high intensity management are: a pre commercial thinning
at age 15 to 680 trees per hectare (275 TPA), followed by a commercial
thinning at age 30 which removes slightly less than a third of the
basal area (to 90 ft2/ acre). The
stand is simulated as having a site index of 122 as consequence
of the management treatments.
Changing the site index is the best way to model the impacts in
the growth associated with fertilization practices in a simulation
(McCarter, April 25th 2002). Growth
projections were simulated for the base and the high intensity to
40 year rotations, growing the high intensity case to a 50-year
rotation is also evaluated. Questions of interest relate to the
assessment of trade offs in terms of carbon and costs: the economics
associated with a higher intensity of management and slightly longer
rotations. In particular, is the additionality between the two prescriptions
worth the time and the investment?
The economic optimization assessment will be examined first, followed
by the carbon analysis at the forest, products, displacement and
substitution levels.
4.2.1 The Economics
The same methods used in the Rotation case are used in this economic
evaluation for the Intensity case, with the same assumptions from
Table 18. All treatment costs are included in the analysis, and
an interest rate of 5% is used to provide SEV comparisons. The prescription
for each scenario includes the costs of a pre commercial thinning
for the mid intensity base case, and the costs of a pre commercial
and commercial thinning with two fertilizations for the high intensity
case. The same prescription will be repeated over time to calculate
the SEV. Results from calculations of SEV are presented in Figure
22.
The economics look best for the 40 year rotation with high intensity
management, with an SEV of $487, or about 5% more than the base,
with an SEV value of $463. The 50 year rotation high intensity is
about 87% of the base case SEV. Despite these differences, in general
terms the economics are relatively insensitive to the different
intensity management strategies and the extension of the rotation
for a few years.
On the other hand, when looking at longer rotations, the SEV value
is dramatically lower, with the 80 year rotation having a value
of $39 (8% of the base SEV), and the 120 year rotation a - $159
SEV value.
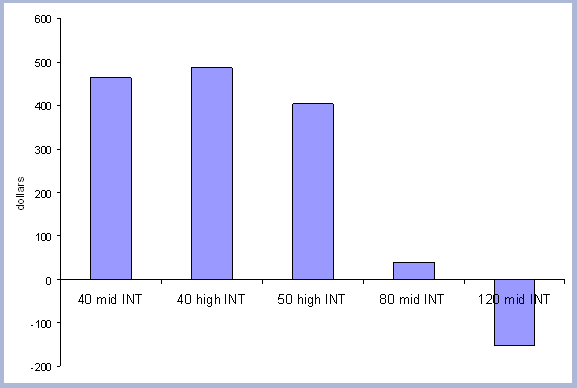 |
| Figure 22. Soil Expectation Value (SEV) for all intensities and all rotations in dollars. |
The carbon storage for the three different intensity cases adds insight into potential benefits. Is the best economical strategy also best on a carbon sequestration basis, or where are the greater gains and through what economic loss (cost) do we get the greatest storage?
4.2.2. The Forest Carbon
Since it is an afforestation case, the starting point is assumed
to be zero in terms of carbon at the sites in year 2000, the beginning
of the management cycle.
| Table 19. Summary of carbon pools for the forest components in metric tons per hectare for the three management prescriptions. | ||||||||||||||||||||||||||||||||
|
When looking at the forest carbon at the end of the rotation (Table 19), the high intensity management scenario has 11.4 metric tons/ ha (or about 7%) less carbon than the base case. Most of this difference might be attributable to the commercial thinning. Ten more years of growth under the intensive management regime are equivalent to 40 more metric tons/ha of standing carbon by the end of the rotation, an 18% increase from the base case scenario. The biggest difference among scenarios is found in the stem component, followed by dead roots and litter. The amount of litter is between 3 to 6% of the forest carbon, but will increase considerably after harvesting, when most of the canopy is assumed to be left scattered on site. The pools that grow the fastest are the stems and the roots in all cases, exemplified below by the 40 high intensity management forest carbon distribution among components (Figure 23).
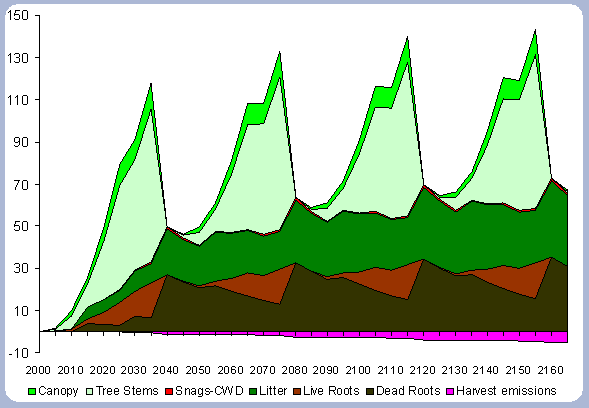 |
| Figure 23. Forest carbon in metric tons/ha through time for the 40 year rotation high intensity management case. |
Forest biomass at the end year of the rotation is not shown because
harvest is assumed in 2040, therefore in 2040 there is no standing
forest, it has been harvested. The litter build up can be appreciated
both in 2015 after the pre commercial thinning, and later in 2030,
with the commercial thinning. Regeneration harvest takes place every
40
years, and it is the building block of the forest floor carbon.
Net forest carbon (forest operation emissions subtracted) for all
intensities is shown in Figure 24, as the area under the curves.
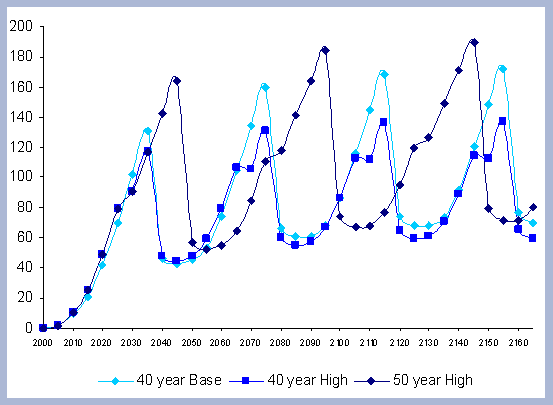 |
| Figure 24. Net forest carbon for all management intensities through time in metric tons/ha. |
All intensities sequester greater amounts of carbon from one rotation to the next, indicating a build up in the dead root and litter components, that remain higher than 50 metric tons/ha after the first rotation and through the 165 years considered. The litter component is part of the net gain from afforestation, maintained through reforestation in time. Another observation is that the difference in forest carbon between the 40 high and base management intensities gets bigger with time, from about 10 metric tons/ha in 2040 to about 40 metric tons/ha in 2160.
The 50 year high intensity management scenario reaches higher levels of forest carbon throughout the 165 years considered. The pattern of carbon accumulation seems to be reaching a steady state for both 40-year intensity management scenarios after the 4th rotation. The 40 base case scenario retains higher levels of carbon from one rotation to the next (more and bigger dead roots and litter), which manifests itself into higher levels of carbon sequestration overall through the management period considered. This level of carbon retained form one rotation to the next catches up with the 50 year rotation high intensity management after about four 40 year base rotations. Averages for the three rotations at different intervals of time are analyzed next, in order to show which management prescription sequesters more forest carbon depending on the window of time considered. Periods encompass forty years and consider the last harvest at years 40, 80, 120 or 160 to occur just after the interval, not before.
Average carbon sequestered is presented for four intervals of time through the management time considered for all intensities in Figure 25. The intervals follow the Rotation Length intervals to be compatible for further comparison, although scales have been adjusted to make patterns and trends more obvious among the closely related scenarios.
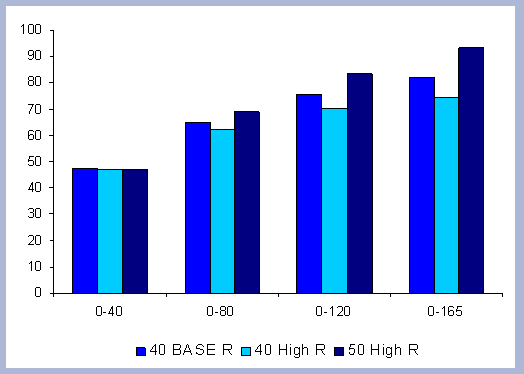 |
| Figure 25. Average net forest carbon for all intensities in four intervals in MT/ha. |
The periodical average of net forest carbon sequestered increases
for all management intensities and rotation lengths through time.
The average goes from about 47 metric tons/ha for all rotations
in the first interval to about 85 metric tons/ha for the 40 base
case and 77 metric tons/ha for the 40 high intensity when considering
the 165 years, with 60 and 45% increase in their averages respectively.
The base case sequestered about 10% more carbon in the forest than
the 40 year high intensity management, but this difference can only
be appreciated after the second 40 year rotation.
As expected, the 50 year rotation sequesters more net average carbon
than any of the two 40 year rotations at any interval of time considered.
The 50 high intensity rotation goes from an average of about 65
metric tons/ ha (first interval) to almost a 100 metric tons/ha
when considering the whole management period, a 53% increase. The
base case sequesters about 85% on average of what the 50 year high
intensity management scenario does.
In summary, the 50 high intensity rotation provides the greatest
carbon sequestration at the forest level on average, followed by
the base case and the 40 high intensity case.
4.2.3 The Carbon in Products
Carbon at the forest level is a very important pool, but there
are other potential pools into which carbon can be stored for long
periods of time. Part of this exercise is to incorporate and assess
the transferring of the standing pools into the product pools and
look at the trade offs (Table 20).
The first thing to notice is that total volumes harvested do not
translate into total carbon sequestered in products. This is due
to the commercial thinnings happening earlier in time than the final
harvest, and those products derived from the intermediate operations
having had time to decompose. Under such context, the base case
sequesters a total of 102 metric tons/ha in products, barely higher
than the total in products for the 40 high management intensity.
However, the base case scenario has at the end of the rotation about
6 % less carbon in the long term product pool. The long term product
pool is decayed at a 1% per year, and it is constituted essentially
of lumber for construction.
| Table 20. Harvesting volumes and total carbon in products for the three rotations. | ||||||||||||||||||||||||||||||||||||||||
|
When addressing the longer rotation scenario, ten more years of
growth are equivalent to 30 metric tons/ha more carbon in products,
with almost 15 metric tons/ha more in long term products. This is
to say that 10 more years of growth are equivalent to about 30%
more on the total average respect to both 40 year rotations, and
about 36% more than the base in the carbon average for the lumber
pool.
4.2.3.1 Carbon Emissions from Forest operations
and Manufacturing
Forest operations and manufacturing emissions, as explained previously,
accumulate through time and must be subtracted from the total carbon
sequestered in the different pools if any evaluation of potential
carbon sequestration is to be balanced in time and space.
The differences in forest operation emissions are negligible among
scenarios. The fertilizer application is included in the total emissions
from forest operations, and it is added at the time of the commercial
thinning (age 30) and ten years previously (age 20). There are barely
any differences either, when looking at averages through time, among
rotations in terms of manufacturing emissions (Figure 26).
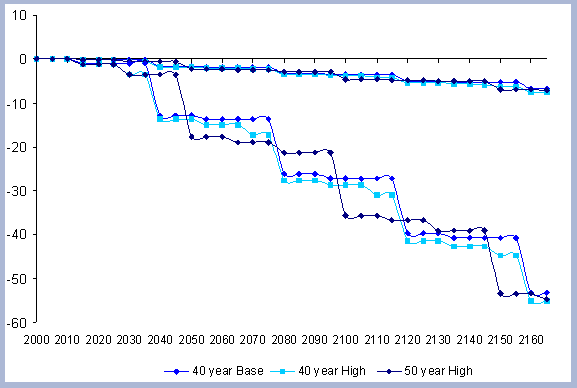 |
| Figure 26. Forest operations
and manufacturing emissions in metric tons/ha of carbon for
all intensities and rotations through time. |
4.2.3.2 Average Net Carbon in Products
Figure 27 shows net total products through the 165 years of management.
The emissions from harvesting and manufacturing operations have
been deducted.
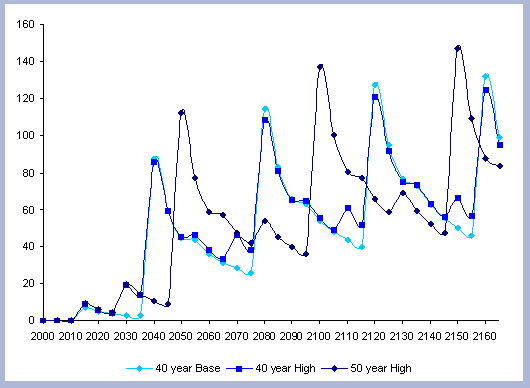 |
| Figure 27. Net products in metric tons/ ha of carbon for all management intensities through time. |
Carbon product pools are higher at time of harvest for the base 40 year rotation, but the 40 high intensity makes up for that difference with the commercial thinning. The total carbon amount in products increases through time for all intensities. Further inquiry into average periodical carbon sequestration is required to establish if ten more years of growth on three rotations make up for four shorter rotations in the 165 years described. Average carbon sequestered in products in different intervals is summed up in the following graph, with net carbon sequestered in the first 40 years, first 80 years, 120 and total for the management scenario (Figure 28). Forty years are considered per interval period assessed and consider the last harvest at years 40, 80, 120 or 160 to have occurred just after the interval, not before.
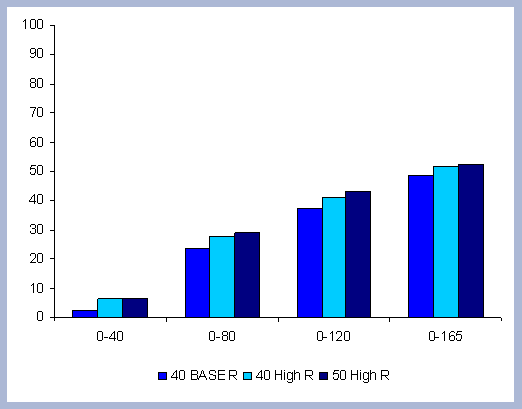 |
| Figure 28. Average periodical carbon sequestered in product pools in four different intervals of time, in metric tons/ ha of carbon. |
Average periodical sequestration of carbon in product pools increases
through time for all intensities considered. The 40 base case scenario
has the smallest amount of carbon in the products pool through time,
at any interval considered. All intensities and rotations reach
levels of above 50 metric tons/ha sequestered on average on a 165
year basis. The big difference observed in the first interval of
40 years is due to the lack of commercial thinning in the Base case
scenario.
The 50 year scenario shown below sequesters the highest amount of
carbon on average in its product pools (Figure 29).
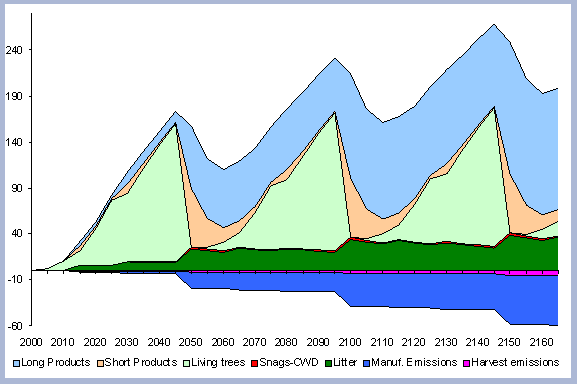 |
| Figure 29. Carbon sequestration in the different pools by category, in metric tons/ha for the 50 high intensity rotation through time. |
On average, the amount of carbon in products from three 50 year
rotations under high intensity management (52.5 Mt /ha ) is roughly
equal to the carbon in products of four 40 year high intensity management
rotations (51.8 Mt/ ha), with the higher intensity cases sequestering
8 and 6% more carbon on average through the 165 years than the base
case scenario.
As stated previously, the product pool is an important pool for
carbon sequestration, and if put to a long term use, carbon can
definitely accrue in time on an average basis. The short term products,
because of a faster decomposition rate, are almost all gone by the
end of the rotations (Figure 29). The long term products accumulate
through time, corresponding to the pattern already observed in Figure
27. In Figure 29, forest operations and manufacturing emissions
can be observed increasing through time, starting from an afforestation
case, for the 50 year high intensity management rotation.
4.2.4. The Carbon in Forest and Products
The next step is to assess trade offs between intensity scenarios
for the forest and product pools of carbon together through time,
shown in Figure 30 as area under the curves. By the end of the 165
years of management, the 50 year rotation high intensity has sequestered
an average of 149 metric tons/ha. This carbon average for the 50
rotation is 11% higher than the average sequestered by the Base
40 year rotation, with 133 metric tons/ha in the combined pools.
The 40 high intensity sequesters the least amount of carbon in the
combined pools, at about 3% less than the base case. The differential
at the products carbon level is not enough for the 40 high intensity
rotation to make up for the less carbon sequestered at the forest
level.
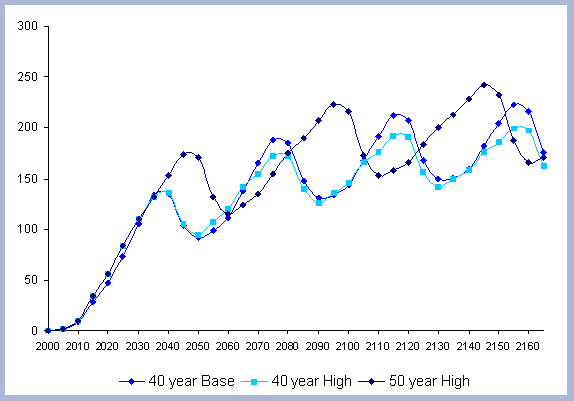 |
| Figure 30. Net forest and net product carbon in metric tons/ha for all intensity scenarios through time. |
All management intensities and rotations increase their carbon pools through time, a logical result of forest and products pools increasing on their own basis. The base case average total pool is always smaller than the 50 year high intensity management, and this average difference increases with time. The base case total average is smaller than the 40 high intensity for the first two intervals, and becomes greater when encompassing more than 120 years as the period of analysis (Figure 31). This change in patterns is mostly attributed to carbon residuals accumulating at the forest floor level after harvest operations.
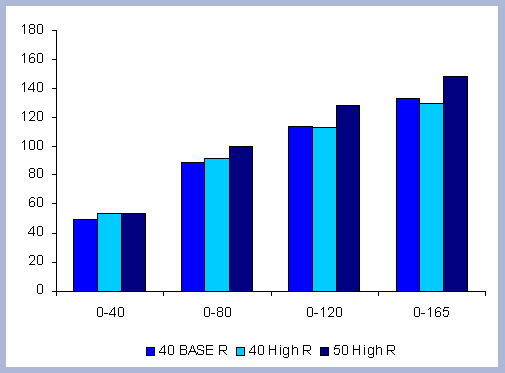 |
| Figure 31. Net average in forest and product pools for all intensities in metric tons/ha at different intervals of time through the 165 years of management. |
Table 21 presents a summary of the carbon pools and their estimates for the different management intensities and rotation lengths evaluated so far. The base case shows the least amount of carbon sequestered in forest products for all interval periods considered, followed by the 40 high and the 50 high rotations which is the greatest both in terms of forest and products carbon. The base case has greater amounts of carbon sequestered at the forest level which gives it higher overall pools later in time (after 120 years) against the 40 high intensity which sees the benefits of the higher amount of carbon in products in the first two intervals assessed. In first interval, the 40 high intensity is 7% greater in forest and products carbon than the base case. This difference is reduced to 3% when looking at 80 years as the interval.
| Table 21. Summary table of averages for the forest, the products and the combined pools at different period intervals for all scenarios in metric tons/ ha. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The pattern is reversed in the third and fourth intervals, with the base case providing greater amounts of carbon than the 40 high intensity. The 50 year high intensity scenario is greater when compared to the base case on all intervals considered, with the difference slightly increasing with the interval of time considered, going from about 7% more than the base in the first interval to about 12% more carbon than the base in forest and products when looking at the 165 years considered.
4.2.5 Carbon displacement
Carbon displacement corresponds to the amount of carbon not emitted
from the burning of fossil fuels when biomass is utilized instead
while producing the same amount of energy. It is displacement and
it is permanent and cumulative through time because fossil fuel
emissions are replaced by non-fossil ones.
The analysis is based on the assumption that all short term products
are used as biofuel in the wood boiler, replacing in such manner
the burning of natural gas, with its correspondent carbon emissions
from manufacturing burdened for the use of the co-products. The
carbon manufacturing emissions representing the lumber production
have only about a 50% burden and therefore need to be adjusted to
100% burden when using the short term products as biofuel. Forest
operation carbon emissions stay the same.
The efficiency of a wood boiler is only 67% compared to an 80% for
the fossil fuel boiler. This lower efficiency, together with the
fact that the short term products are at a 50% moisture (wet basis)
make the amount of carbon not emitted, meaning displaced from fossil
fuels, about half the carbon sequestered in short term products
right after the regeneration harvest, on a metric tons/ha basis.
However, as stated previously, the displacement is permanent and
cumulative through time while short term products decompose rapidly
according to our assumptions (1%/ year on average). This property
can be observed in Figures 32 for the 40 high intensity management
scenario through the 165 years of management considered in the analysis.
The long term products (light blue area) are shown stacked together
with the displacement carbon (purple area) and the carbon in the
short term products is shown as the black line.
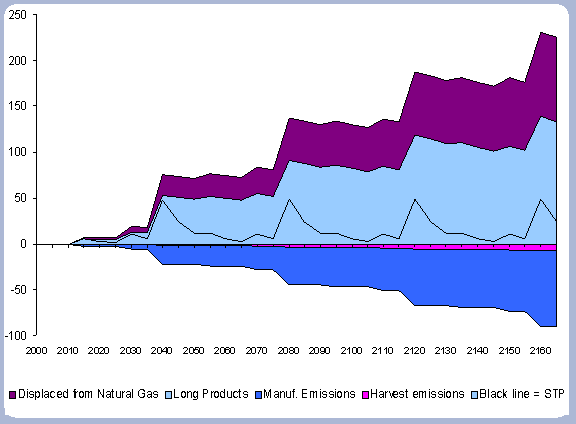 |
| Figure 32. Carbon displacement against short and long terms products through time in metric tons of carbon /ha for the 40 high intensity management scenario. |
The short term products and the displacement carbon do not exist
at the same time, because the energy for the displacement comes
from the burning of the totality of the short term products pool.
Figure 32 is simply illustrating how the short term products pool,
although greater than displacement on a 1:1 ratio, is almost totally
gone through decomposition by the end of the rotation, while the
carbon displaced keeps accumulating in time, becoming a net benefit
in the overall carbon sequestration story by the time of the second
rotation in this particular case.
Figure 33 shows the carbon displacement for all the management intensities
through the scenario of 165 years considered.
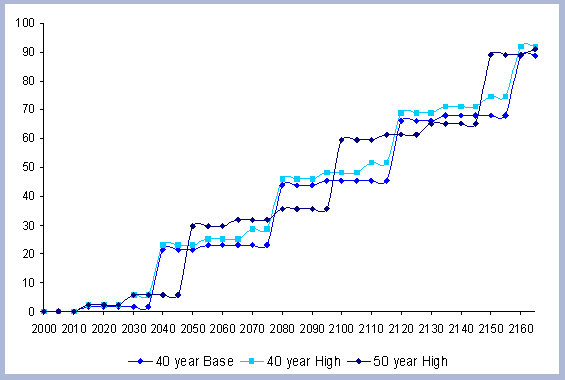 |
| Figure 33. Carbon displaced from natural gas by burning biomass for all intensity rotations in metric tons/ha through time. |
The base 40 rotation displaces 35.5 metric tons/ ha on average
through the 165 years considered. The 50 high intensity rotation
displaces an average of 37.68 metric tons/ha or 6% more carbon than
the base, followed by the 40 high intensity rotation with 8% more
carbon than the base displaced on average e, with 38.32 metric tons/
ha.
The displacement carbon does not change the overall pattern of the
carbon sequestration differences among intensities and rotations.
Table 22 shows the carbon average estimates when adding the displacement
carbon to the already considered carbon averages in the forest and
product pools in metric tons/ha.
| Table 22. Summary of averages for the forest, the products and the combined pools with displacement at different period intervals for all scenarios in metric tons/ ha. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Displacement is a positive contribution to the carbon sequestration
story in the long run, with higher levels of carbon sequestered
on average for all intensities and rotations considered in the Intensity
Management Case.
When considering the forty year interval, on average the amount
of carbon sequestered is greater when using the short term products
for something other than energy. When considering the 80 year interval,
the total carbon pool is on average about the same with the displacement
for all intensities and rotations than without the displacement,
following the patterns described previously. However, when the interval
is 120 years or more, the displacement has accumulated through time,
proving to be a positive contribution to the carbon sequestration
story in the long run.
The base case is still sequestering more carbon on average than
the 40 high intensity management scenario when considering the 165
years of management, with the difference between the two slightly
smaller than when no displacement was considered (2% instead of
3%). The 50 high intensity scenario is on average about 10% greater
than the base case.
Figure 34 shows the development of the carbon pools for the 50 year
high intensity management scenario that has shown so far the greatest
average levels of carbon sequestration.
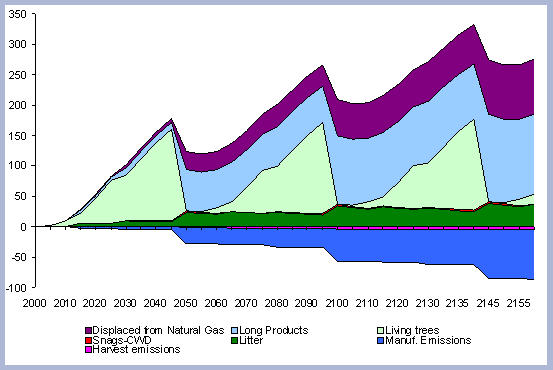 |
| Figure 34. Carbon sequestered in the different pools through time for the 50 high intensity management in metric tons/ha. |
4.2.6 Carbon substitution
As explained previously in the Methods, when no wood is harvested
on the hectare, there are less wood framed houses / ha and more
steel framed houses built / ha. This construction trade-off represents
the basis for the substitution numbers. If wood is harvested at
any point in time, wood will be substituting for steel and more
wood framed houses will be built on the hectare, representing fewer
emissions.
The number of potential wood houses from the regeneration harvest
volumes ranges from 3 for the 40 high intensity management scenario,
meaning minus 2 steel houses built per hectare, to up to 5 wood
houses for the 50 high intensity management, equivalent to minus
4 steel houses built on the hectare, with the differential in carbon
emissions associated to this different type of construction (Table
23).
| Table 23. Changes in the number of wood and steel houses for different intensity rotations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The substitution factor dramatically changes the carbon sequestration story. Relative to the Base 40 rotation net forest and products carbon by including substitution at the house level, the 40 high intensity rotation always shows more sequestration than the base 40 rotation, even though before substitution came into play, the situation was the opposite (Figure 35).
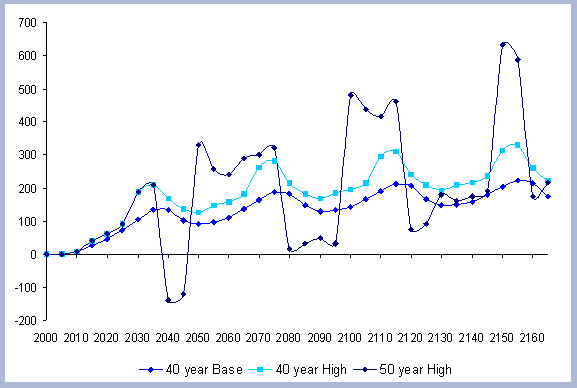 |
| Figure 35. The 40 mid intensity rotation as base line (medium blue) for the substitution analysis against the other two cases, in metric tons/ha of carbon sequestered through the 165 years considered. |
Because the rotation lengths are the same, and the only thing that changes is the intensity in the silvicultural management, it is readily observable that slightly higher volumes in harvesting and thinning are equivalent to great reductions in the amounts of carbon emissions when considering substitution of high fossil manufacturing materials. In the case of the 50 high intensity rotation, although starting positive, it goes negative in 2040 when the base and shorter rotations are harvested, and slowly recovers to positive levels again after 2050, when the first regeneration harvest occurs for that management scenario.
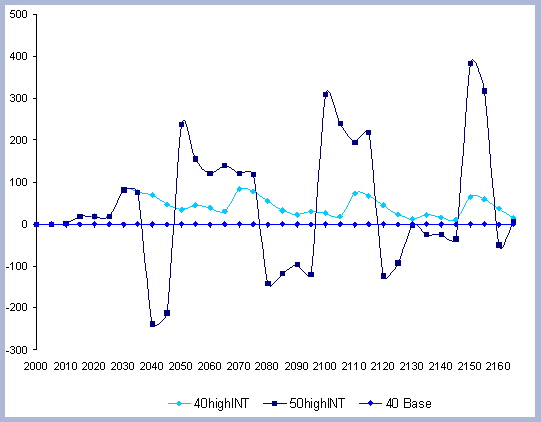 |
| Figure 36. Same picture as Figure 35, but with the Base 40 normalized to zero. |
When the base line is normalized to zero (Figure 36), the carbon differential among the base and the 40 high intensity management can be observed to diminish through time, even though the 40 high intensity management is still considerably above the base throughout the 165 years considered. The longer rotation shows a positive trend overtime.
Figure 37 shows average carbon against the base rotation for the four intervals of time previously considered when addressing effects of substitution within the carbon sequestration story. The base case is now the smallest on average carbon sequestration at any interval of time, while before addressing substitution it was only smaller in the short run. When considering the 165 years of management, the 40 and 50 high intensity management scenarios sequester about 38% and 43% more carbon respectively than the base. Less fossil fuels were used by the wood substitution provided by these scenarios.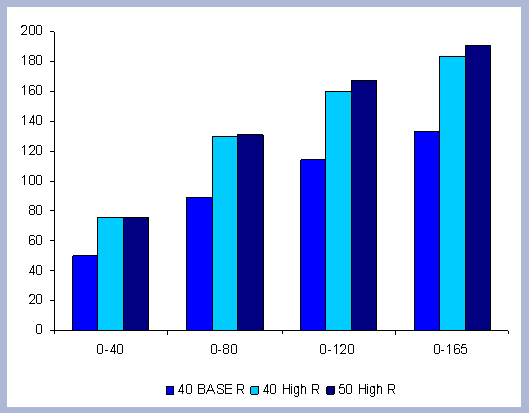 |
| Figure 37. Average carbon sequestration differentials against the base 40 with average forest and products carbon, when incorporating substitution in metric tons/ha for the defined interval of time. |
The 40 and 50 high intensity management scenarios are very close in terms of their carbon sequestration potential when addressing all the components considered in the carbon story in the first two intervals with no additionality from the 10 extra years of growth in the short time. However, the last two intervals show an additionality from the 50 high intensive rotation of about 7 metric tons/ha on average.
Table 24 shows the summary of the carbon story, at the forest, products, displacement and substitution levels.| Table 24. Summary table of averages for the forest, the products and the combined pool with displacement and substitution at different period intervals for all scenarios in metric tons/ ha. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
So far, the carbon story has only been addressed at the standing or products level. If only looking at those two pools, the 40 high intensity makes more sense in terms of the carbon pool sequestered and the economics of the investment. It is followed by the 50 high intensity, where 10 more years of growth are shown to provide significant increases in the carbon pools at a modest economic cost. The carbon story does not change this pattern when incorporating the displacement carbon. When considering the substitution effects, slightly higher levels of carbon sequestration are provided with the 50 high intensity rotation than the 40 high intensity rotation, with slightly poorer economic returns.
5. DISCUSSION
The discussion section will be addressed in two parts. First, the carbon model and its assumptions will be assessed with regards to the quality and reliability of the outputs and potential places for improvement. Secondly, the results from the Rotation and Intensity case scenarios will be explored looking for the greatest potential for sequestration and reduction of emissions, while addressing the economic trade offs of the different alternatives of management explored.
5.1 The Carbon Model
The significance of the carbon model created for the Pacific Northwest region lies in that all of the components considered important in the carbon sequestration story are incorporated in it, and all are based on outputs from a specific forest stand. In this way, forest carbon, products carbon, displacement and substitution carbon issues as well as the economic tradeoffs can be evaluated by the landowner and incorporated in the management strategy whether based on objectives that are local (jobs, regulations, economics) and/or global (land use changes, environment, global warming).
5.1.1 The Forest Module
The Forest Module is a first step in developing a close carbon
model for the Pacific Northwest region. Even though it is considered
a closed model, there are components that need to be addressed in
the future and their contribution to the carbon cycle reassessed.
For example, we are not addressing the understory component, and
justifying it because of the small amount of biomass relative to
the overall stand and difficulties in the modeling of its development.
In their study on a series of Douglas-fir stands, Turner and Long
(1975) found that even though the understory is less than 5% of
the total standing biomass, it was a significant portion of the
total stand productivity (up to 17%) and even a higher proportion
of the organic matter returned to the forest floor (up to 43% of
the total return). Changes in site productivity are not addressed
in the model but it is understood that site productivity is not
a fixed nor given attribute and the understory component might be
a critical element of that productivity in its interaction and contributions
to the carbon soil.
The model assumes that the entire crown is left on site after harvest,
and none of the material is burned as site preparation for the regeneration
planting. Leaving the slash on the ground is beneficial for nutrient
cycling and avoidance of immediate emissions.
These assumptions might have to be revised to be closer to the reality
of the management in the Pacific Northwest forest. Although those
practices would be ideal for the maintenance of the forest floor
component, they can be counter productive in the regeneration process.
The way decomposition is calculated for the different components in the carbon model is not related to moisture nor temperature changes within the stand, even though the literature suggests they are directly correlated. Harvesting usually increases decomposition rates of the forest floor material because it causes higher soil temperatures and moisture (Aber et al 1978). Temperature and moisture variables have been found to be the main factors explaining decomposition patterns (Gholtz et al 2000). These variables can change considerably in the development of a stand, especially after harvesting or thinning operations. The model does not account for these changes at the stand scale and the simple way in which the model is estimating decomposition carbon losses to the atmosphere could be refined.
There is also the issue of scale. There is carbon loss from an individual tree as it decomposes (i.e. snags) however, if looked at as a group of snags, they still result in accumulation of carbon storage over time, even though the individuals are decomposing (Harmon 2001). There is not a single scale that is correct for the evaluation of carbon, but caution needs to be exerted when looking at the addition of outputs at the stand level, because some of these might not be directly transferable to a higher scale of assessment, such as in the detritus component.
Although forest inventory data may provide the best available information on carbon accumulation at the forest stand level, it is not enough to calculate total forest ecosystem carbon. An ecosystem-level carbon model should integrate changes in detritus and soil carbon together with the tree carbon changes, as well as land use change data to be able to better assess, understand and predict net carbon storage in managed and disturbed forest ecosystems.
5.1.2 The Products Module
The products module is largely based on the CORRIM (2002) interim
report "Life Cycle Environmental Performance of Renewable Building
Materials in the Context of Residential Building Construction Substitution".
Some of the assumptions within specific appendixes in the Report
are still being refined. Until the Report is peer reviewed, some
of the inputs and outputs used in the products module might continue
to change.
For example, the long term storage products within the product module
of the carbon model does not consider plywood even though it represents
more than 25% of the total production in the five manufacturing
plants surveyed in the Pacific Northwest.
In the lumber assessment, there is potential for error in the material
balance when going from conversion of wood volume (ft3)
given by LMS volume output tables to the log scale volume (Scribner)
required for the use of the outputs given by the mills. This conversion
is dependent on log sizes, and as of now, the products stream of
outputs is solely based on volume, without addressing the diameter
of the logs and the sensitivity of this conversion. The value of
larger logs that yield higher lumber grades and have higher monetary
and plausible mechanical values are ignored in the model. Diameter
is used to determine the volume of sawlogs available.
Issues with regards to the manufacturing emissions can be customized
for specific mills. The initial case assumed essentially no use
of residuals for energy production.
5.2. The Case Scenarios
5.2.1 The Rotation Case
When considering carbon sequestration alternatives and the possible
questions to explore, the idea that longer rotations might be more
beneficial in the accounting of carbon than shorter rotations was
inevitable. Most of the literature seems to convey this point of
view, by addressing the carbon at the forest level in longer rotations
versus shorter rotations, which is readily obvious because of the
greater biomass produced, or indirectly, by arguing issues of old
growth conversion to younger stands (Cooper 1983, Harmon et al 1990,
Dewar 1991, Schultze et al 2000).
The study results show this to be true for carbon at the forest
level, but not when product flows including substitute products
are included. The carbon patterns observed at the forest level are
reversed when looking at substitution issues. The carbon sequestration
losses from substituting other materials for wood more than offsets
the increase carbon storage in the forest at least for the first
several hundred years, a period exceeding normal carbon targeting.
These issues have been mostly ignored by the literature (Harmon
et al 1990, IPCC 2000). The IPCC report on land use addresses long
term sink capacity. It states that "additional carbon can be
stored in an ecosystem only if more carbon is kept for the same
period of time or the same amounts of carbon are kept over longer
periods of time", with harvesting negatively impacting these
increased sinks. Again, the IPCC statement is true when addressing
forest level sink issues. The statement does not provide an overall
vision for the role of wood in the substitution scheme within the
carbon cycle and all of its components.
From a policy perspective, providing carbon credits for longer rotations
will not make much sense and might even be counterproductive unless
the targets of interest are hundreds of years in the future. Credits
for longer rotations, because of the tradeoffs in the substitution
of wood by more fossil and energy intensive manufactured products,
can become detrimental when considering the full analysis. However,
using a carbon tax that differentially affects fossil fuel consumption
would be a potential way to deter individuals, communities and industries
from using fossil fuel intense wood substitutes, and thus reduce
the amount of carbon emissions.
The Rotation case analysis showed that the hypothesis that long
rotations are good both for biodiversity and carbon is not true
when looking at the full carbon assessment. The Rotation case also
showed the significant contribution of afforestation in the carbon
accounting, which has been often suggested by the literature (UNFCC
1998, Chomitz 2000, Birdsay et al 2000). Moreover, harvesting afforested
lands will sequester even more carbon through product pools than
not harvesting. Even though no management can sequester more carbon
at the forest level, it is counterproductive by not producing renewable
wood products that will substitute for fossil fuel intensive products.
The benefits of the afforestation activities gave rise to the idea
for a carbon credit on afforestation. If afforestation can be estimated
and monitored through time, the idea of implementing a carbon credit
on afforestation seems plausible benefiting the landowner while
providing incentives to avoid land use change, which more usually
than not result in deforestation.
Figure 38 shows the average carbon sequestered in forest and products
pool on a 40 year case scenario. The average indicated by the red
line in the figure is about 90 metric tons/ha over the 80 years
considered. If $5 are paid per metric ton of carbon, the combination
of this incentive plus the SEV return from forestry alone would
double the landowner's return producing a high motivation to keep
land in forestry or convert other land with a low return to forestry.
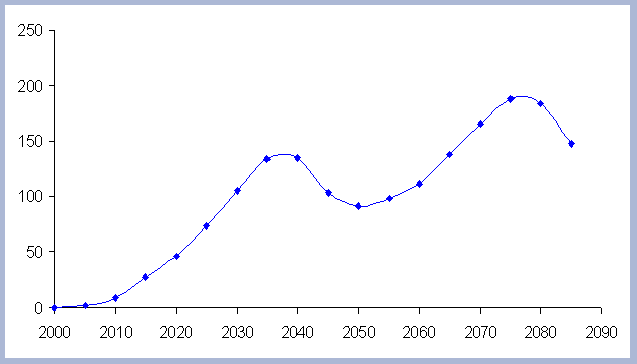 |
| Figure 38. Carbon in forest and product pools for Base 40 year rotation in metric tons/ha through 80 years from afforestation through reforestation. |
The biodiversity value of longer rotations cannot be ignored. When
values and objectives other than carbon override the full carbon
assessment scheme, such as habitat and biodiversity concerns, long
rotations might be appropriate.
From this analysis it is clear that mitigation of carbon emissions
depends on the extent to which fossil fuel use is displaced by biofuel
and wood products. Hence the full assessment developed here is important.
5.2.2. The Intensity Case
The intensity case looked at differentials in carbon pools at the
forest, products, displacement and substitution levels when managing
a stand to different intensities. The idea was to be able to define
where additionality might occur, if from higher levels of thinning
and fertilization, or from slightly longer rotations.
One would expect that more intensive management would produce more
sequestration in the products pools. The results show that while
thinnings reduce the carbon in the forest, the gain in carbon product
flows which can substitute for fossil intensive products, more than
offset that loss at the forest level.
This can be observed in figure 39 where significant differences among intensity management regimes can be found in the amount of carbon sequestered. When considering the first 40 year interval (1), there are about 26 more metric tons/ha of carbon in the intensive 40 and 50 year rotations than in the base case. This differential increases to about 30 when looking at the 80 year interval (2). There is no differential at that point in time attributed to additionality between the 40 and 50 high intensity rotations.
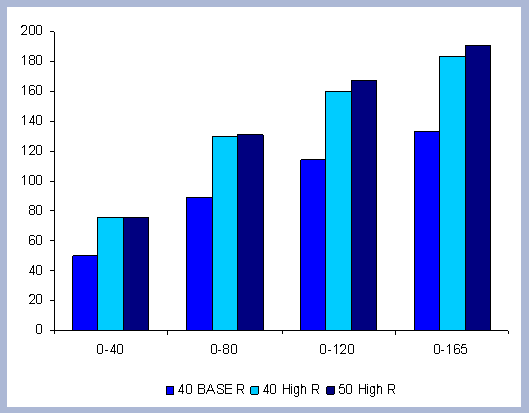 |
| Figure 39. Carbon pools in forest, products when substitution is considered, for all intensities at different intervals of time through the management scenario. |
Intervals 3 (0-120 years) and 4 (0- 165 years) show carbon additionality between the 40 high and 50 high intensity rotation. While extending the rotation slightly (i.e. 10 years) sequesters somewhat more carbon, the economic loss to the owner to produce about 7 more metric tons/ha of carbon would require a comparable carbon credit. This credit should be more or less equal to the differences in SEV divided by number of additional acres, in this case 83 / 7 = $12. The target is still deemed to be too far out in time to be of consideration.
5.3 Closing remarks
While the displacement of fossil fuels by burning short lived products
for energy increases carbon sequestration since the displacement
is permanent and does not decay like short lived products, the low
efficiency in the conversion results in little short term benefit.
After several rotations the increase sequestration can become significant.
However, the length of time required would appear to be beyond useful
carbon credit targets.
The model demonstrates the level of complexity needed to adequately
account for carbon sequestration related to forest management. Carbon
credit systems will have to be very complex not to induce unintended
consequences (i.e. emissions from renewable vs. non renewable resources).
Increasing afforestation and intensifying forest management are
possible objectives. While longer rotations have many environmental
benefits, such as biodiversity and habitat for endangered species,
they are more likely to produce substitution from fossil fuels than
increased carbon storage in the short term.
A general increase in the cost of fossil fuels or a tax will encourage
both afforestation and intensive management. As little as $ 5 /
metric ton of carbon was shown to double the expected land value
for the high intensive rotation case. This should be enough of an
incentive to convert marginal agricultural lands to forest land.
5.4. Future work
A complete sensitivity analysis should be done to evaluate specific
products and their end use. The decaying functions should be more
complex to represent more accurately the decomposition of the forest
components and the life of the forest products. When reliable impacts
of management regimes on soil carbon become available they should
be added to the model.
Given the interest in carbon credit incentives, it could be important
to assess in what situations an incentive might contribute to afforestation
without causing deforestation somewhere else. Perez- Garcia (1994)
showed that market incentives produce at least partially offsetting?.
Furthermore, it will be important to assess when and under what
conditions substitution would not be the model in which to base
the full analysis for carbon sequestration.
6. BIBLIOGRAPHY
- Anonymous. 1992. Text of United Nations Framework Convention on Climate Change, UNEP/WMP Information Unit on Climate Change, Climate Change Secretariat, Palais des Nations, Geneva, Switzerland, 29pp.
- Aber, J.D, D.B. Botkin and J.M.. Melillo. 1978. Predicting the effects of different harvesting regimes on forest floor dynamics in northern hardwoods. Can. J. For. Res.8: 306-315.
- Aber J.D, J.M Melillo and C.A McClaugherty (1990). Predicting long term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Canadian Journal of Botany 68: 2201-2208.
- Alaback, P.B, 1982. Dynamics of understory biomass in Sitka spruce-western hemlock forests of southeast Alaska. Ecology 63 : 1932-1948.
- Alaban, D.H, and D.A. Perla. 1990. Impact of timber harvesting on soils. Pp 377-391. In: S.P Gessel, D.S. LacCate, G.F. Weetman, and R.F. Powers (eds). Sustained Productivity of Forest Soils. 7th North American Forest Soils Conference, University of British Columbia, Vancouver, BC.
- Apps, M.J. and D. Price. 1996. Forest Ecosystems and the Global Carbon cycle: Introduction. In: Forest Ecosystems, Forest Management and the Global Carbon Cycle. NATO ASI Series Vol I 40. Eds. Apps and Price. Springer-Verlag Berlin Heidelberg.
- Aztet, T. , R.F Powers, D.H. McNabb, M.P. Amaranthus, and E.R. Gross.1989. Maintaining long-term forest productivity in southwest Oregon and Northern California. Pp 185-201. In: D.A. Perry, R. Meurisse, B. Thomas, R. Miller, J. Boyle, J. Means, D.R. Perry, and R.F. Powers (eds), Maintaining long-term productivity of Pacific Northwest Forest Ecosystems. Timber Press, Inc. Portland, Oregon
- Bergman R and J. Zerbe. 2001. Primer on Wood Biomass for Energy. Technology Marketing Unit. Forest Products Laboratory. USDA Madison Wisconsin. www.fpl.fs.fed.us/tmu.
- Binkley, D., K. Cromack, and R.L. Fredriksen. 1982. Nitrogen accretion and availability in some snowbrush ecosystems. Forest Sci. 28:720-724.
- Binkley, D.1983. Ecosystem production in Douglas-fir plantations: Interactions of read alder and site fertility. For. Ecol. Management.5 :215-227.
- Birdsay, R.A. 1992. Carbon storage and accumulation on United States forest ecosystems. USDA Forest Service General Technical Report. WO-59.
- Birdsay, R.A. 1992. Changes in Forest Carbon storage from increasing Forest Area and Timber Growth. In: Forests and global change: Forest management opportunities for mitigating carbon emissions (vol. 2) eds. R.N Sampson and D. Hair, 1-26. Washington, DC: American Forests.
- Birdsay, R.A.1996. Carbon storage for major types and regions in the conterminous United States. In: Forests and global change: Forest management opportunities for mitigating carbon emissions (vol. 2) eds. R.N Sampson and D. Hair, 1-26. Washington, DC: American Forests.
- Birdsay, R.A and L.S Heath. 1997. The forest carbon budget for the United States. In USDA Forest Service Global Change Research program highlights: 1991-1995. eds. R. Birdsay, R. Mickler, D. Sandberg, R. Tinus, J. Zerbe and K. Obrien. General technical report, NE- 237. Washington, DC:USDA Forest Service.
- Birdsay, R.A, R. Alig and D. Adams. 2000. Mitigation activities in the Forest Sector to Reduce Emissions and Enhance Sinks of Greenhouse gases. USDA Forest Service Gen. Tech. Report. RMRS-GTR-59.
- Brady, N.C. and Ray R. Weil. 1996. The Nature and Property of Soils. Prentice Hall Ed. New Jersey.
- Briggs, D. 1994. Forest products measurements and conversion factors, with special emphasis on the U.S. Pacific Northwest. Institute of Forests Resources Contribution No. 75. Seattle: University of Washington College of Forests Resources.
- Bodegom, van A.J., H.J.F. Savenije, G. van Tol. 2000. The Challenge of Including Forests as sinks within the Clean Development Mechanism. Theme Studies Series 4. Forests, Forestry and Biological Diversity Support Group. . IAC- EC LNV.
- Bormann, B.T., P.S. Homann, L. Bednar, M.A. Cairns, J.R. Barker. 1998. Field Studies to Evaluate Stand-Scale Effects of Forest Management on Ecosystem Carbon Storage. Interagency Agreement DW 12936179. Programmatic Plan. Carbon budget databases. Design and evaluation.
- Bray, J.R. and E.Gorham. 1964. Litter production in forests of the world. Advances in Ecological Research 2:101-157.
- Bunce, R.G.H. 1968. Biomass and production of trees in a mixed deciduous woodland. Girth and height as parameters for estimation of tree dry weight. Journal Ecology 56: 759-775.
- Burshel, P., E. Kursten, B.C. Larson, M. Weber. 1993. Present role of German forests and forestry in the national carbon budget and options to its increase. Water, Air, and Soil Pollution 70, 325-349.
- Canary, J.D., R.B. Harrison, J.E Compton, and H.N. Chappell. 2000. Additional carbon sequestration following repeated urea fertilization of second growth Douglas-fir stands in Western Washington. Forest Ecology and Management 138 : 225-232.
- Chen H., M. Harmon, R. Griffiths. 2001. Decomposition and nitrogen release from decomposing woody roots in coniferous forests of the Pacific Northwest: a chronosequence approach. Can. J. For. Res. 31: 246-260.
- Chomitz, K.M. 2000. Evaluating carbon offsets from forestry and energy projects: How do they compare? Prepared for the Development Research Group. World Bank.
- Cissel, J.H, F.J. Swanson, and P.J. Weisberg. 1999. Landscape Management Using Historical Fire Regimes: Blue River, Oregon. Ecological Applications, 9(4), pp 1217-1231.
- Cooper, C.F. 1983. Carbon storage in managed forests. Can. J. For. Res. 13:155-166.
- CORRIM. 2002. Interim report. "Life Cycle Environmental Performance of Renewable Building Materials in the Context of Residential Building Construction Substitution" Phase I.
- Covington, W.W. 1981. Changes in forest floor organic matter and nutrient content following clear cutting in northern hardwood. Ecology 62: 41-48.
- Cropper, W.P. Jr., and K.C. Ewel. 1984. Carbon storage patterns in Douglas-fir ecosystems. Can. J. For. Res 14:855-859.
- Curtis, R.O. 1997. The role of extended rotations. In: Kohm, K.A., and J.F. Franklin. (Tech. ed.) 1997. Creating a forestry for the 21st century: the science of ecosystem management. Washington, DC: Island Press. pp. 165-170.
- Davis, L.S and K.N. Johnson. 1986. Financial Analysis and The Arithmetic of Interest. In: Forest Management. 3rd Edition. The McGraw-Hill Series in Forest Resources.
Delcourt, H.R. and W.F. Harris. 1980. Carbon budget of the southeastern United States: analysis of historical change in trend from source to sink. Science 210: 310-323.- Dewar, R.C. 1990. A model of carbon storage in forests and forest products. Tree physiology 6:416-28.
- Dewar, R.C. 1991. Analytical model of carbon storage in trees, soils, and wood products of managed forests. Tree Physiology 8:239-258.
- Dewar, R.C., M.C.R. Cannell. 1992. Carbon sequestration in trees, products and soils of forest plantations: an analysis using U.K. examples. Tree physiology 11, 49-71.
- Dimock, E.J. 1958. Litter fall in a young stand of Douglas-fir. Northwest Science 32: 19-29.
- Edmonds, R.L. 1979. Decomposition and nutrient release in Douglas-fir needle litter in relation to stand development. Can. J. For. R. 9: 132-140.
- Edmonds, R.L. 1980. Litter decomposition and nutrient release in Douglas-fir, red alder, western hemlock, and Pacific silver fir ecosystems in western Washington. Can J. For. Res. 10: 327-337.
- Edmonds, R.L. 1987. Decomposition rates and nutrient dynamics in small diameter woody litter in four ecosystems in Washington, USA. Can. J. For. Res. 17: 499- 509.
- Faustmann, M., (1849) Calculation of the Value which Forest Land and Immature Stands Possess for Forestry. Trans. in Gane, M. (ed.), (1968) Martin Faustmann and the Evolution of Discounted Cash Flow. Institute paper 42, Commonwealth Forestry Institute, University of Oxford.
- Ford, D.E., and R.O. Teskey. 1991. The concept of closure in calculating carbon balance of forests: accounting for differences in spatial and temporal scales of component processes. Tree Physiology 9, 307-324.
- Fernandez, I.J., J. Logan, and C.J. Spencer. 1989. The effects of site disturbance on the mobilization and distribution of nutrients and trace metals in forest soils. Environmental Studies Center, University of Maine, Orono.
- Franklin, J.F and C. T. Dyrness. 1973. Natural vegetation of Oregon and Washington. USDA Forest Service General Technical Report PNW8.
- Franklin, J.F., D.R. Berg, D.A. Thornburgh, J.C. Tappeiner. Alternative Silvicultural Approaches to Timber Harvesting: Variable retention Harvest Systems. In: Kohm, K.A., and J.F. Franklin. (Tech. ed.) 1997. Creating a Forestry for the 21st century: the science of ecosystem management. Washington, DC: Island Press. pp. 165-170.
- Franklin Associates. 1998. Combustion of Wood in Industrial boilers. SimaPro5 Life-cycle Assessment Software Package, version 36, 2001.
- Fujimori, T., S. Kawanabe, H. Saito, C.C. Grier, and T. Shidei. 1976. Biomass and net primary production in forests of three major vegetation zones of the northwestern United States. J. Jap. For. Soc. 58: 360-373.
- Fung, I. 1994. The global carbon cycle and the atmospheric record: "The problem definition". In: Forest Ecosystems, Forest Management and the Global Carbon Cycle. Eds. M.J. Apps and D.T. Price. NATO ASI Series I: Global Environmental Change vol. 40. Eds. Apps and Price. Springer-Verlag Berlin Heidelberg.
- Gardner, R.H and J.B. Mankin. 1981. Analyses of biomass allocation in forest ecosystems of the IBP. In: Dynamic properties of forest ecosystems. Ed. D.E. Reichle. Cambridge University Press, Cambridge, pp. 451-497.
- Gessel, S.P and J. Turner. 1976. Litter production in western Washington Douglas-fir stands. Forestry 49, 63-72.
- Gholtz, H.L., D. Wedin, S. Smithermna, M.E. Harmon, and W.J. Parton. 2000. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Global Change Biology 6: 751-765.
- Gholtz, H.L. and R.F. Fisher.1982.Organic matter production and distribution in slash pine (Pinus elliotii) plantations. Ecology 63: 1827-1839.
- Gholtz, H.L. 1982. Environmental limits on aboveground net primary production, leaf area, and biomass in vegetation zones of the Pacific Northwest. Ecology 63: 469-481.
- Gholz, H.L., C.C. Grier, A.G. Campbell, and A.T.Brown. 1979. Equations for estimating biomass and leaf area of plants in the Pacific Northwest. Forest Research Lab, Oregon State University, Corvallis, Oregon.
- Grace, J., M. Rayment. 2000. Respiration in the balance. Nature 404: 819-820.
- Grier, C.L., D. W Cole, C.T Dyrness and R. L. Fredriksen. 1974. Nutrient cycling in 37 and 450 year old Douglas fir ecosystems. In: Integrated research in the coniferous biome. Edited by R. H. waring and R. L. Edmonds. Coniferous Forest Biome Bull. No 5, University of Washington, Seattle. Pp 21-34.
- Grier, C.L. and R.H. Waring 1974. Conifer foliage mass related to sapwood area. Forest Science 20:205-206.
- Grier, C.L. 1975. Wildfire effects on nutrient distribution and leaching in a coniferous ecosystem. Can J. For. Res. 5:599-607.
- Grier, C.C, and R.S.Logan.1977. Old -growth Pseudotsuga menziesii communities of a western Oregon watershed: biomass distribution and production budgets. Ecological Monographs 47:373-400.
- Hansen J., I. Fung, A. Lacis, D. Rind, G. Russel, S. Lebedeff, R. Reudy, P. Stone. 1988. Global climate changes as forecast by GISS's three-dimensional model. J. Geophysical Resources 93 : 9341-9364.
- Harmon, M. 2001. Carbon Sequestration in Forests: Addressing the Scale Question. Journal of Forestry 99 (4): 24-29.
- Harmon M., O. Krankina, J. Sexton. 2000. Decomposition vectors: a new approach to estimating woody detritus decomposition dynamics. Can. J. For. Res. 30: 76-84.
- Harmon, M.E., and J. Sexton. 1996. Guidelines for measurements of woody detritus in forest ecosystems. U.S. LTER Publication No 20. University of Washington, Seattle, WA 73pp.
- Harmon, M. E., J. M. Harmon, W.K. Ferrel, and D. Brooks. 1996. Modeling carbon stores in Oregon and Washington forest products: 1900-1992. Climate Change 33: 521-550.
- Harmon, M.E., and J.J. Lee. 1995. A carbon budget for forests of the conterminous United States. Ecological Applications, 5(2), pp. 421-436.
- Harmon, M.E. 1993. Woody debris budgets for selected forest types in the US. In: The forest sector carbon budget of the United States: carbon pools and flux under alternative policy options. Corvallis, OR: US EPA/600/3-93/093. pp: 151-178.
- Harmon, M.E. 1992. Long-term Experiments on the Log decomposition at the H.J. Andrews Experimental Forest. General Technical Report 280. USDA FS PNW-GTR-280
- Harmon M.E, W.K. Ferrel, J.F Franklin. 1990. Effects on carbon storage of conversion of old-growth forests to young forests. Science 247:699-702.
- Harmon, M.E., J. F. Franklin, F.J. Swanson, P. Sollins, J.D. Lattin, N.H. Anderson, S. V. Gregory, S.P. Cline, N/G. Aumen, J.R. Sedell, G.W. Lienkaemper, K. Cromack Jr, and K.W. Cummins. 1986. The ecology of coarse woody debris in temperate ecosystems. Recent Advances in Ecological Research 15: 133-302.
- Harris W.F., R.A. Goldstein, G.S. Henderson. 1974. Analysis of forest biomass pools, annual primary production and turnover of biomass for a mixed deciduous forest watershed. Contribution No. 119 from the Eastern Deciduous Forest Biome, US-IBP.
- Haynes R.W., R.J. Alig, and E. Moore. 1994. Alternative simulations of forestry Scenarios Involving Carbon Sequestration Options: Investigation of Impacts on Regional and National Timber Markets. USDA. Pacific Northwest Research Station. General Technical Report 335. PNW-GTR-335.
- Haynes R.W and J.F. Weigand. 1997. The Context for Forest Economics in the 21st Century. In: Creating a Forestry for the 21st Century. Eds. K.A. Kohm and J.F. Franklin. Island Press, Washington DC.
- Houghton, R.A., J.E. Hobbie, J.M. Melillo, B. Moore, B.J. Peterson,
G.R. Shaver and G.M. Woodwell. 1983. Changes in the carbon content
of terrestrial biota and soils between 1860 and 1980: a net release
of CO2 to the atmosphere. Ecol. Monogr. 53: 235-262.
- Hunt E.R. Jr., F.C. Martin, and S.W. Running. 1991. Simulating the effects on climatic variation on stem carbon accumulation of a ponderosa pine stand: comparison with annual growth increment data. Tree Physiology 9, 161-171.
- Huntington, T.G., and D.F Ryan. 1990. Whole-tree harvesting effects on soil nitrogen and carbon. For Ecol. Management 31;193-204.
- Johnsen, K.H., D. Wear, R. Oren, R.O teskey, F. Sanchez, R. Will, J. Butnor, D. Markewitz, D. Richter, T. Rials, H.L. Allen, J. Seiler, D. Ellsworth, C Maier, G. Katul, and PM Dougherty. 2001. Meeting global policy commitments: Carbon sequestration and Southern Pine Forests. Journal of Forestry 99 (4).
- Intergovernmental Panel on Climate Change. 1990. Working Group III. Climate change: Formulation of response strategies. Island press, Washington DC.
- IPCC Workshop statement for Carbon Balance on World's Forested Ecosystems: Towards a Global Assessment, Proc. Intergov. Panel on Climate Change Workshop, Joensuu, Finland, 11-15 May 1992. Publications of the Academy of Finland.
- Intergovernmental Panel on Climate Change. 2000. Land use, Land Use Change, and Forestry: A Special Report of the IPCC, Cambridge University Press, Cambridge, UK.
- Johnson W.C, D.M. Sharpe. 1983. The ratio of total to merchantable forest biomass and its application to the global carbon budget. Can. J. For. Res. 13: 372-383.
- Johnson, C.E., A.H. Johnson, T.G. Huntington, and T.G. Siccama. 1991. Whole-tree clear-cutting effects on soil horizons and organic matter pools. Soil Sci. Soc. Amer. J. 55:497-502.
- Johnson, D.W.1992. Effects of forest management on soil carbon storage. Water, Air, and Soil Pollution 64:83-120.
- Johnson, D.W.1992. Effects of forest management on soil carbon storage. NCASI Technical Bulletin No 628.
- Johnson, L. 2001. CORRIM Forest Resources Report - Harvest Factors (unpublished) Personal Communication. March 2002.
- Keegan, Ch., C. Fiedler and F. Stewart.1995. Cost of timber harvest under traditional and "new forestry" silvicultural prescriptions. Western Journal of Applied Forestry, 10(1):36-41.
- Kellogg, L.D., P. Bettinger and R. Edwards.1996. A comparison of logging planning, felling and skyline yarding costs between clearcutting and five group-selection harvesting methods. Western journal of Applied Forestry, 11(3):90-96.
- Keyes, M.R. 1979. Seasonal patterns of fine roots biomass, production and turnover in two contrasting 40 year old Douglas-fir stands. Can .J. For. Res. 11:599-605.
- Keyes, M.R., and C.C. Grier. 1981. Above- and below-ground net production in 40-year-old Douglas-fir stands on low and high productivity sites. Can. J. For. Res. 11: 599-605.
- King, J.E. 1966. Site index curves for Douglas-fir in the Pacific Northwest. Weyerhaeuser Forest. Paper. No 8.
- Koch, Peter. 1991. Wood vs. non-wood materials in U.S. residential construction: Some energy related international implications. CINTRAFOR WOP 36. College of Forest Resources, University of Washington, Seattle, WA.
- Kohlmaier, G.H, M. Weber, R.A. Houghton. 1998. Carbon Dioxide Mitigation in Forestry and Wood Industry. Eds. Kohlmaier, Weber and Houghton. Springer-Verlag Berlin Heidelberg.
- Kuiper, L. C. and Coutts, M. P. 1992. Spatial disposition and extension of the structural root system of Douglas-fir. For. Ecol. Management, 47:111-125.
- Lal, R 1995. Carbon sequestration in soils. In Soils and Global Change: R.Lal, J.M Kimble, E. Levine and B.A. Stewart Eds. CRC/Lewis Publishers, Boca Raton, FL.
- Landsberg, J.J., M.R. Kaufman, D. Binkley, J. Isebrands and P.G. Jarvis. 1991. Evaluating progress toward closed forest models based on fluxes of carbon, water and nutrients. Tree Physiology 9: 1-15.
- Long, J.N., J. Turner. 1975. Aboveground biomass of understory and overstory in an age sequence of four Douglas-fir stands. Journal of Applied Ecology 12: 179-188.
- Macadam, A.M. 1987. Effects of broadcast slash burning on fuels and soil chemical properties in the sub-boreal spruce zone of central British Columbia. Can. J. For. Res. 17:1577-1584.
- Marland G, K. Fruit and R. Sedjo. 2001. Accounting for sequestered carbon: the question of permanence. Environmental Science & Policy 4: 259-268.
- Marland G. and S. Marland. 1992. Should we store carbon in trees? Water, Air and Soil Pollution 64: 181-195.
- McArdle, Richard E, Walter H. Meyer, and Donald Bruce. 1949. The Yield of Douglas Fir in the Pacific Northwest. Technical Bulletin No. 201, United States Department of Agriculture, Washington, D.C. 74 p.
- McCarter, J. April 25th 2002. UW. Personal interview on simulation assumptions for fertilizers.
- Moore B. and B.H Braswell III. 1994. Planetary metabolism: understanding the carbon cycle. Ambio 23: 4-12.
- Musselman, R.C. and D.G. Fox. 1991. A review of the role of temperate forests in the global CO2 balance. J. Air waste Management Association. 41:798-807.
- Neff, J.C, G.P. Asner. 2001. Dissolved Organic Carbon in Terrestrial Ecosystems: Synthesis and a Model. Ecosystems 4 : 29-48.
- Oliver, C.D., A. Osawa and A. Camp. 1998. Forest dynamic and resulting animal and plant population changes at the stand and landscape levels. Journal of Sustainable Forestry 6: 281-312.
- Oliver, C.D., and B.C. Larson. 1996. Forest stand dynamics. Update edition. John Wiley & Sons. New York, NY.
- Oliver, C.D. 1993. What is wood quality, how is it achieved, and why is it important? In: Special Paper (SP) 15, CINTRAFOR (The Center for International Trade in Forest Products), College of Forest Resources, University of Washington, Seattle WA.
- Oliver, C.D. 1992. A landscape approach: Achieving and maintaining biodiversity and economic productivity. Journal of Forestry 90: 20-25.
- Oliver, C.D., J.A. Kershaw, Jr. and T.M. Hinckley. 1990. Effects of harvest of old growth Douglas-fir and subsequent management of carbon dioxide levels in the atmosphere. In: Are forests the answer? Proceedings of the Society of American Foresters National convention, Silviculture Working Group, 29 July- 1 August 1990.
- Oliver, C.D. 1981. Forest development in North America following minor disturbances. Forest Ecology and Management, 3:153-168.
- Pacala, S.W., G.C. Hunt, R.A. Houghton, R.A Birdsay, L. Heath, E.T. Sundquist, R.F. Stallard, et al. 2001. Convergence of U.S carbon flux estimates from inventories of ecosystems and inversions of atmospheric data, Science 292: 2316-2320.
- Perez- Garcia, J. 1994. An Analysis of Proposed Domestic Climate Warming Mitigation Program Impacts on International Forest Products Markets. WP50 (26 pp) CINTRAFOR (The Center for International Trade in Forest Products), College of Forest Resources, University of Washington, Seattle WA.
- Post, W.M., W.R. Emmanuel, P.J. Zinke and A.G. Stangenberger. 1982. Soil carbon pools and world life zones. Nature 298:156-159.
- Raich, J.W. 1983. Effects of forest conversion on the carbon budget of a tropical soil. Biotropica 15 : 177-184.
- Raich, J.W and K.J. Nadehoffer. 1989. Below ground carbon allocation in forest ecosystems. Ecology 70: 1346-1354.
- Reichle, D.E, B.E. Dinger, N.T. Edwards, W.F. Harris and P.Sollins. 1973. Carbon flow an storage in a woodland ecosystem. In Carbon and the biosphere. Edited by G.M. Woodwell and E.V Pecan. AEC Symp. Ser. No 30 tech. Inf. Cent. Oak Ridge, TN. CONF-720510 pp. 345-365.
- Row, C. and R.B. Phelps. 1996. Wood Carbon flows and storage after timber harvest. In: Forests and global change: Forest management opportunities for mitigating carbon emissions (vol. 2) eds. R.N Sampson and D. Hair, 27-58. Washington, DC: American Forests.
- Row, C. and R.B. Phelps. 1996.Effects of selected forest management options on carbon storage. In: Forests and global change: Forest management opportunities for mitigating carbon emissions (vol. 2) eds. R.N Sampson and D. Hair, 59-90. Washington, DC: American Forests.
- Ryan, M.G. 1991. A simple method for estimating gross carbon budgets for vegetation in forest ecosystems. Tree Physiology 9, 255-266.
- Sampson, R.N. 1997. Forest and wood products role in carbon sequestration. Presented at the International Climate Change Conference & Technologies Exhibition, Baltimore, Maryland (EPA- American Forests Cooperative Agreement CR-820797-01-0).
- Santantonio, D. Hermann, R.K. and Overton, W.S. 1977. Root biomass studies in forest ecosystems. Pedabiologia, Bd. 17:1-31.
- Santantonio, D. and R.K Hermann,1985. Standing crop, production, and turnover of fine roots on dry, moderate and wet sites of mature Douglas-fir in western Oregon. Ann Sci. For. 42: 113-142.
- Schlamadinger B., and G. Marland. 1996. The role of forest and bioenergy strategies in the global carbon cycle. Biomass and Bionenergy 10:275-300.
- Schlamadinger B., and G. Marland. 2000. Land Use and Global Climate Change: Forests, Land Management and the Kyoto Protocol. Pew Center on Climate Change, Arlington, VA, USA, p 54 available at www.pewclimate.org.
- Schlesinger, W.H. 1977. Carbon balance in terrestrial detritus. Ann. Rev. Ecol. Syst 8: 51-81.
- Schneider, S.H. 1989. The Changing Climate. Scientific American 261: 70-79.
- Schultze, E-D., C. Wirth, and M. Heimann. 2000. Managing forests after Kyoto. Science 289: 2058-2059.
- Simpson, L.G., D.B. Botkin, R.A.Nisbet. 1993. The potential aboveground carbon storage of North American Forests. Water, Air, and Soil Pollution 70: 197-205.
- Skog, K., G.A. Nicholson. 1998. Carbon cycling through wood products: the role of wood and paper products in carbon sequestration. Forest Products Journal 48 (7/8): 75-83.
- Smith, D.M, B.C. Larson, M.J. Kelty, P.M.S.Ashton. 1996. Stand development and Structure. In: The Practice of Silviculture: Applied Forest Ecology. Ninth Ed.John Wiley and Sons, Inc.
- Snell, J.A.K and J.K Brown. 1978. Comparison of tree biomass estimators-dbh and sapwood area. Forest Science 24_455-457.
- Spies, T.A.,J.F. Franklin, and T.B. Thomas. 1988. Coarse woody debris in Douglas-fir forests of western Oregon and Washington. Ecology 69:1689-1702.
- Sprugel, D.G. 1985. Changes in biomass components through stand development in wave-regenerated balsam fir forests. Canadian Journal of Forest Research, 15:269-278.
- Turner, J. J. N. Long. 1975. Accumulation of organic matter in a series of Douglas-fir stands. Canadian Journal of Forest Research 5: 681-690.
- Tadaki, Y. 1966. Some discussion on the leaf biomass of forests stand and trees. Bull. Gov. For. Exp. Stn. Tokyo 184:135-161.
- Turner, J and J.N. Long. 1975. Accumulation of organic Matter in a series of Douglas-fir stands. Can. J. for. Res. 5: 681-690.
- Turner, D.P, G.J. Koerper, M. Harmon and J.J. Lee 1995. A Carbon Budget for Forests of the Conterminous United States. Ecological Applications 5(2): 421-36.
- United Nations Framework Convention on Climate Change (UNFCCC). 1998. The Kyoto Protocol to the United Nations Framework on Climate Change. UNFCCCC Document FCCC/CP/1997/7/Add.1. Available at www.unfccc.de
- Vogt, K.A, R.L. Edmonds, G.C. Antos and D.J. Vogt. 1980. Comparison between carbon dioxide evolution, ATP concentrations and decompsootion in red alder, Douglas-fir, western hemlock and Pacific silver fir ecosystems in western Washington. Oikos 35: 72-79.
- Vogt, K.A, C.C. Grier, D.J. Vogt. 1986. Production, turnover, and nutrient dynamics of above and belowground detritus in world forests. Adv. Eco. Res. 15: 303-377.
- Vogt, K.A, D.J. Vogt, E. Moore, B. Fatuga, M. Redlin and R. Edmonds. 1987. Conifer and angiosperm fine-root biomass in relation to stand age and site productivity in Douglas-fir forests. Journal of Ecology 75:857-870.
- Vogt, K. 1991. Carbon budgets of temperate forest ecosystems. Tree Physiology 9, 69-86.
- Wayburn, L.A et al. 2000. Forest carbon in the United States:
Opportunities & Options
for private lands. Prepared for the Pacific Forest Trust. - Webber, B.D. 1977. Biomass and nutrient distribution patterns in a young Pseudotsuga menziesii ecosystem. Can. J. For. Res. 7: 326-334.
- Webster, S. 11.15.02. WSU. Personal interview on time rates for fertilization.
- Wilson, J. 2002. Exported Results from SimaPro for air emissions from the production of one Mbf of planed dry lumber (unpublished). Personal communication.10/17/2002.
- Winjum, J.K., S. Brown, B. Schlamadinger. 1998. Forest harvest and wood products: sources and sinks of atmospheric carbon dioxide. Forest Science 44(2): 272-284.
- Woudenberg,S.W and T.O. Farrenkopf 1995. The Westside Forest Inventory Data Base: User's Manual, USDA Forest Service, Intermountain Research Station, INT GTR-317.